Signalment:
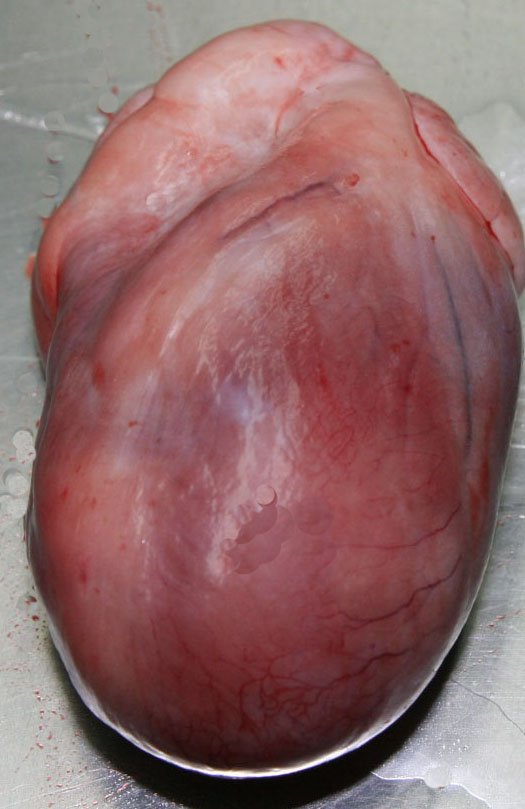
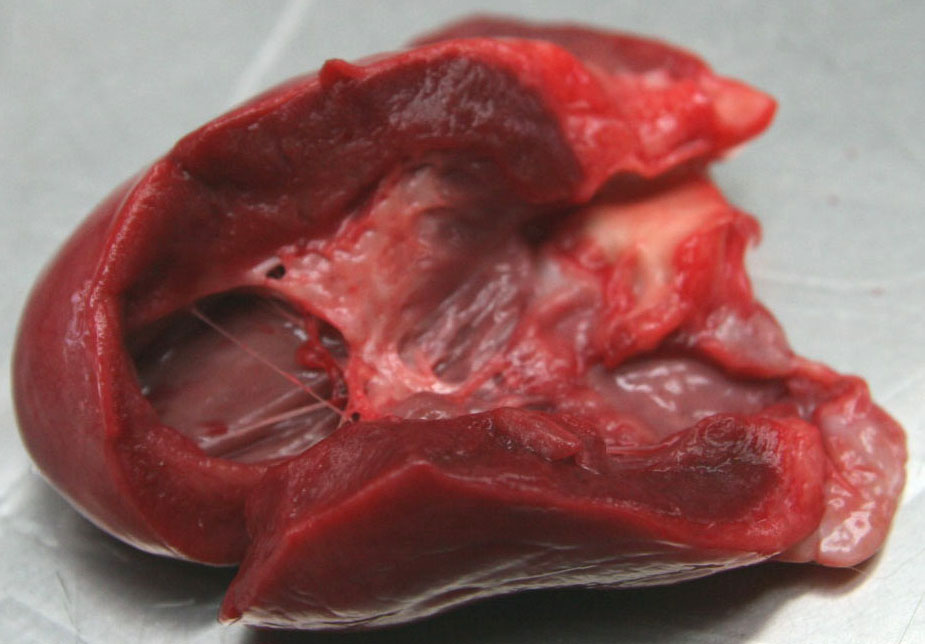
Gross Description:
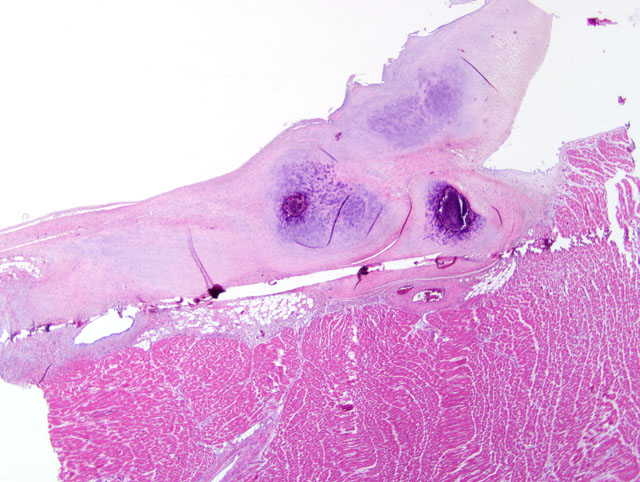
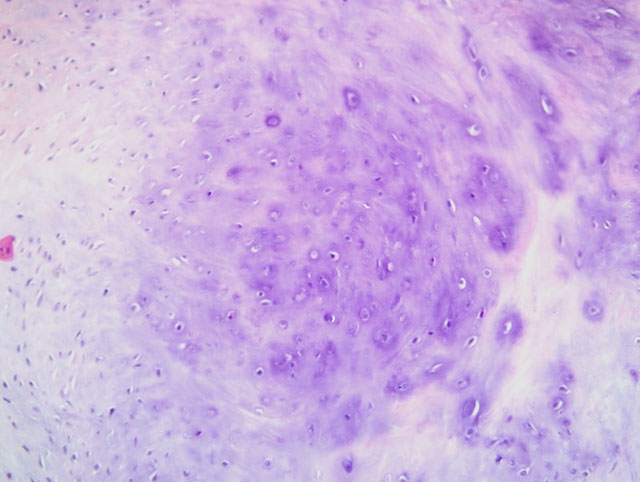
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Cats with RCM present with diastolic dysfunction and increased myocardial stiffness. The etiology is unknown.(3) Two basic forms of RCM in man have been reported: myocardial and endocardial RCM. These two forms are also described in the cat. The feline myocardial form, a myocardial disease with restrictive filling and severe atrial dilatation, is idiopathic. The endomyocardial form, endomyocardial fibrosis (EMF), is characterized by marked fibrosis of endocardium and endomyocardium. The etiology of EMF is not known but different backgrounds have been discussed. In human RCM, associations with hypereosinophilic syndrome, endomyocarditis or vasculitis have been suggested.(1) Many cases of human EMF have been reported from the tropics; hence a search for infectious or nutritional causes has often been pursued.
Familial HCM has been reported in Maine coon cats.(4) However, the lack of severe concentric hypertrophy and atrial dilatation is not consistent with a diagnosis of HCM in this case. An association with endomyocarditis and left ventricular endocardial fibrosis has been reported in the cat.(6) The lack of active inflammation in the present case does not rule out a viral or immune-mediated endomyocardial injury with secondary reparative fibrous tissue formation. The ultrasonographic, macroscopic and microscopic findings in the present case are compatible with a diagnosis of restrictive cardiomyopathy of endomyocardial fibrous type. This was based on the presence of many fibrous bands crossing between the papillary muscles and the septum wall. Secondary lung edema with hydrothorax had also developed.
JPC Diagnosis:
Conference Comment:
| Feline Cardiomyopathies | |||
|---|---|---|---|
| Classification | Key Gross Findings | Key Microscopic Findings | Summary |
| Hypertrophic (HCM) | Cardiomegaly with either symmetric concentric ventricular hypertrophy or asymmetric hypertrophy of the LV and a slit-like LV lumen; LA dilation; +/- atrial thrombi, multifocal ventricular fibrosis, endocardial fibrosis in the LV outflow tract | Hypertrophied myofibers with vesicular nuclei; myofiber disarray in the LVFW and IVS; diffuse interstitial fibrosis (particularly in the inner aspect of the LFFW); focal endocardial fibrosis | Most common CM in cats; autosomal dominant inheritance in Maine coon and American shorthair cats; impaired diastolic ventricular filling (due to diminished LV lumen) with usually normal systolic function; distinct from hyperthyroidsm, which also may cause concentric myocardial hypertrophy |
| Restrictive (RCM) |
Severe endocardial thickening; mural thrombosis; marked LA dilation +/- thrombosis; thickened LVFW with diminished LV volume | Replacement of myocardium and endocardium by granulation tissue and fibrosis; histiocytic, and/or lymphoplasmacytic endomyocarditis | Includes the myocardial form and the endomyocardial form; also termed "LV endocardial fibrosis", which may be preceded by endomyocarditis, of which Bartonella sp. has been suggested as a cause; occurs primarily in older cats; impaired diastolic ventricular filling with usually normal systolic function |
| Dilated (DCM) |
All heart chambers enlarged; ventricles dilated, flaccid, and thin-walled; atrophy of papillary muscles and trabeculae; +/- subendocardial fibrosis | Usually minor microscopic lesions; moderate myofiber hypertrophy with mild, diffuse interstitial fibrosis; +/- myofiber loss and replacement with fibrosis; usually no myofiber disarray | Distinct from and rare in comparison to taurine-deficiency myocardial failure (TDMF), which causes identical gross and microscopic lesions and systolic dysfunction resulting in bilateral congestive heart failure |
| Excessive moderator bands (false tendons) |
Numerous pink-white bands spanning the LV lumen and papillary muscles, usually connecting the cranial and caudal papillary muscles to the IVS; LV dilated or thickened | Abnormal moderator bands composed of Purkinje cells and dense collagen covered by endothelium; LV myocyte atrophy or hypertrophy; fibrosis; intramural coronary arterial intimal thickening, medial hyperplasia, perivascular fibrosis, and luminal narrowing | Occurs in mature cats but is considered a congenital defect; clinical signs of left-sided congestive heart failure |
| LV = left ventricle; LVFW = left ventricular free wall; IVS = interventricular septum; CM = cardiomyopathy | |||
Cardiomyopathy is a diagnosis of exclusion and, by strict definition, should be used only to describe idiopathic primary myocardial disease. Therefore, myocardial dilation due to taurine deficiency (i.e. taurine-deficiency myocardial failure [TDMF]) and concentric hypertrophy attributed to hyperthyroidism are distinct from dilated and hypertrophic cardiomyopathies, respectively. In cats with HCM, DCM, RCM, and myocarditis, the detection of feline panleukopenia virus genome by PCR suggests a possible causal role, but a definitive etiology for these conditions remains elusive.(5)
As alluded to by the contributor, this case may represent the chronic sequel of feline endomyocarditis, which can ultimately progress to left ventricular endocardial fibrosis, one of several idiopathic feline cardiovascular diseases grouped together clinically as RCM.(5,6) Some conference participants considered primary endocardial fibroelastosis (EFE) in the differential diagnosis; this congenital anomaly is exceedingly rare in domestic species, and is best documented in Burmese kittens, in which the condition results in microscopically detectable endocardial lymphedema at as early as one day of age. After 20 days of age, diffuse endocardial deposition of collagen and elastin is evident grossly; this may progress to involve Purkinje fibers, which undergo degeneration. Endocardial or myocardial inflammation are not features of EFE.(5)
The moderator emphasized the importance of echocardiography in correctly classifying feline cardiomyopathies clinically. Gross examination at necropsy is often less rewarding than antemortem echocardiography, and microscopic evaluation may be even less helpful, as evidenced by the overlap in microscopic findings between the various cardiomyopathies listed above and the usual paucity of significant microscopic lesions in feline DCM, specifically. A common sequel, observed in up to one third of affected cats, is unilateral or bilateral hindlimb ischemia due to iliac thromboembolism.(5,6)
References:
2. Ferasin L, Sturgess CP, Cannon MJ, Caney SMA, Gruffydd-Jones TJ, Wotton PR: Feline idiopathic cardiomyopathy: a retrospective study of 106 cases (1994-2001). J Feline Med Surg 5:151-159, 2003
3. Fox PR: Endomyocardial fibrosis and restrictive cardiomyopathy: pathologic and clinical features. J Vet Cardiol 6:25-31, 2004
4. Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, Towbin JA: Familial hypertrophic cardiomyopathy in Maine coon cats: an animal model of human disease. Circulation 99:3172-3180, 1999
5. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 17-18, 36, 44-47. Saunders Elsevier, Philadelphia, PA, 2007
6. Stalis IH, Bossbaly MJ, Van Winkle TJ: Feline endomyocarditis and left ventricular endocardial fibrosis. Vet Pathol 32:122-126, 1995