Wednesday Slide Conference, Conference 11, Case 2
Signalment:
1.2-year-old, intact female, German shepherd canine.
History:
This dog was adopted recently from a rescue in Texas. She had a history of a persistent, productive cough that worsened over time. She presented to the UWVC Small Animal Internal Medicine Service for a possible megaesophagus work up. The owner also reported occasional hemoptysis at home and hyporexia. Computed Tomography (CT) revealed a cranial mediastinal mass. Over time, her symptoms worsened and she returned to the hospital due to increased respiratory effort. Thoracic radiographs revealed aspiration pneumonia, atelectasis and confirmed the previously diagnosed static cranial mediastinal mass, which was associated with a segmental megaesophagus and compression of the trachea and primary bronchi. An endotracheal wash performed and showed neutrophilic and eosinophilic inflammation with no bacterial growth. A fine needle aspirate was taken from the mediastinal mass and showed evidence of neutrophilic and eosinophilic inflammation with rare linear material that was suggestive of fungal hyphae. Heartworm and blastomycosis antigen testing and coccidiosis titers were all negative. This dog continued to decline and euthanasia was elected.
Gross Pathology:
A locally extensive portion of the esophagus, beginning from midway down the neck and
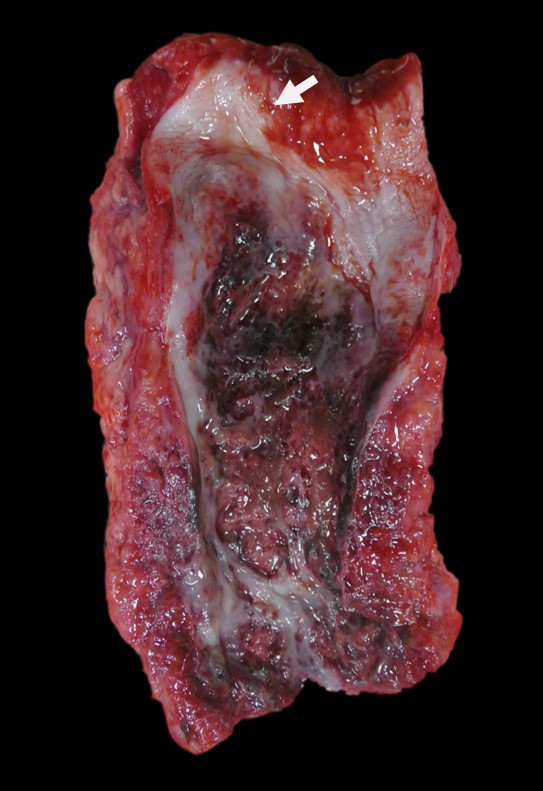
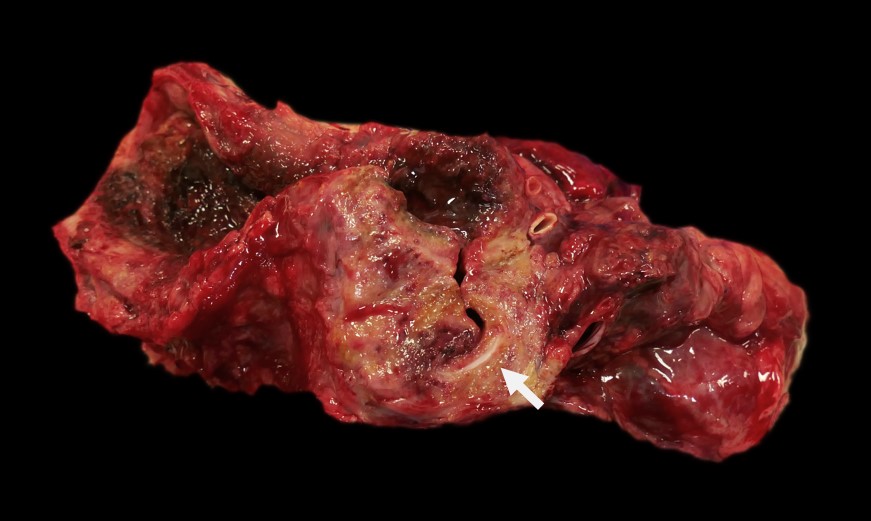
extending to the diaphragm, is markedly thickened, nodular and firm. In this region, the wall of the esophagus is massively expanded, up to 1.5 cm, by abundant firm, tan, fibrous material interspersed with well demarcated pockets of friable reddened tissue with a green tinge (see Figure 1). This change extends beyond the wall of the esophagus to incorporate the adjacent enlarged cranial mediastinal and tracheobronchial lymph nodes, the trachea and the adjacent dorsal portions of the lungs (see Figure 2). The luminal diameter of the trachea is significantly reduced. Within the most severely affected areas, the trachea is almost completely effaced by this process and the tracheal lumen has a significantly distorted and reduced diameter. The mucosal surface of the esophagus is friable and multifocally covered by plaques of stringy friable material (fibrin). Multifocally, there are variably sized areas where the mucosal epithelium is absent (ulcer), including a locally extensive 2.5 x 2 cm region.
Laboratory Results:
A panfungal PCR with sequencing identified Cladosporium species.
Microscopic Description:
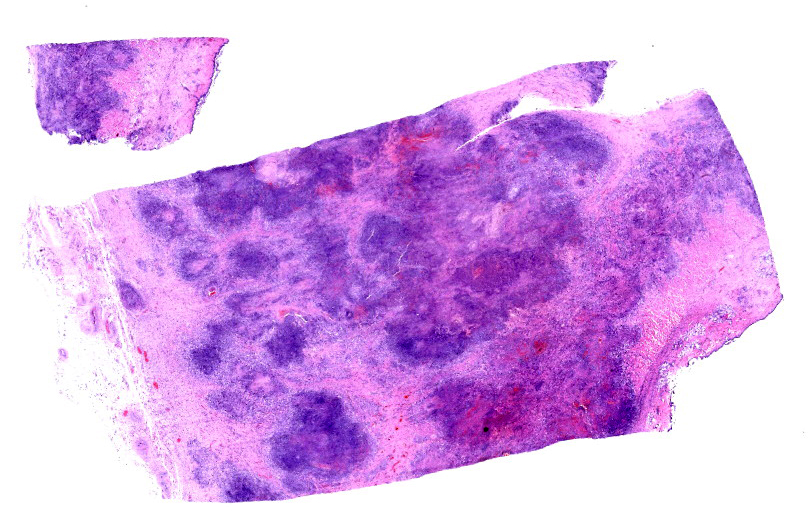
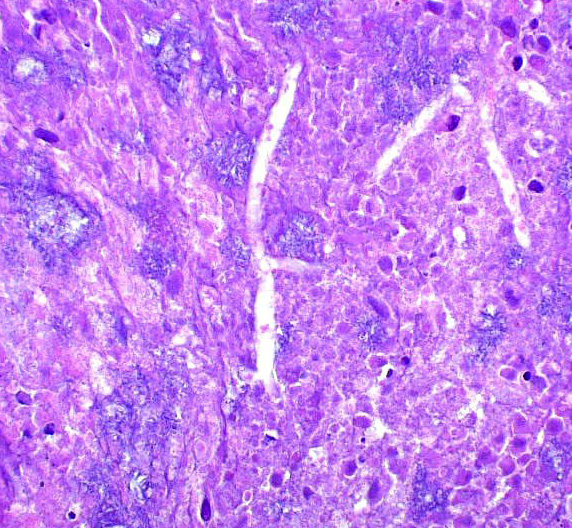
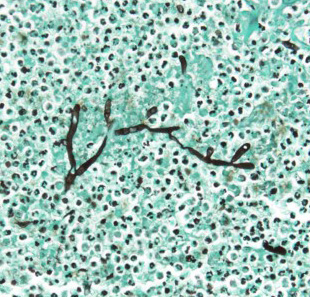
Esophagus (per submitter): Severely expanding and effacing up to 90% of the submucosa are multifocal to coalescing pyogranulomas composed of a central region of amorphous eosinophilic material (necrosis) admixed with cellular and karyorrhectic debris, surrounded by a ring of intact and fragmented neutrophils, epithelioid macrophages and fewer multinucleated giant cells, and further surrounded by a thin rim of lymphocytes and plasma cells (see Figure 3). Within the central areas of necrosis and rarely within the cytoplasm of multinucleated macrophages are many poorly staining, 7 to 10 µm hyphal organisms with thin non-parallel walls, irregular branching, infrequent septation, and rare bulbous dilations up to 15 µm in diameter (see Figure 4). The hyphal organisms are prominent and exhibit strong dark black staining with a Grocott’s Methenamine Silver (GMS) stain (see figure 6) and lack melanin (confirmed with a Fontana Masson’s stain). Intersecting between granulomas and further expanding the submucosa are abundant multifocal to coalescing regions of fibrosis and to a much lesser extent hemorrhage, with a few associated macrophages laden with variably sized, golden-brown, intracytoplasmic pigment (hemosiderin). A few granulomas are centered on or extend to adjacent small to medium caliber blood vessels. Affected vessels are often lined by a few necrotic endothelial cells that are occasionally sloughed into the lumen with the tunica intima partially replaced by variable amounts of fibrin and infiltrated by minimal numbers of similar inflammatory cells (fibrinoid necrosis; vasculitis). Within the adventitia, medium to large caliber vessels are frequently surrounded by moderate to large amounts of perivascular fibrosis.
Contributor’s Morphologic Diagnosis:
Esophagus: Severe chronic multifocal to coalescing and pyogranulomatous esophagitis with intralesional hyphal organisms
Contributor’s Comment:
Given the microscopic features, gross lesions, and morphology of the hyphal organisms, an infection with Pythium sp. or Lagenidium sp. is considered the cause of disease in this animal despite the results of PCR and sequencing, which identified Cladosporium sp. from the formalin fixed tissue. In particular, the microscopic features of this fungus are not consistent with this result and we do not believe that Cladosporium species are the source of infection in this animal.
Morphological features consistent with this case and Pythium/Lagenidium spp. include non-parallel walls, infrequent septa and irregular branching.3,5 A few bulbous dilatations within hyphal structures were noted in examined sections which is more consistent with Cladosporium sp. as well as the irregular branching and infrequent septa; however, other morphological characteristics of Cladosporium sp. include thicker non-parallel walls and apparent pigmentation, which were not evident.11 In addition, Cladosporium sp. frequently exhibits pigmentation, seen either in routine H&E staining or with the aid of a Fontana Masson’s stain, which was not seen in this case. Based on the morphological characteristics, we suspect the hyphal structures are likely Pythium sp. or Lagenidium sp. regardless of PCR results.
Despite the few morphological similarities shared between Pythium/Lagenidium spp. and true fungi, Pythium and Lagenidium spp. are oomycetes (water molds), not fungal organisms. Key differences between oomycetes and fungal organisms include the lack of chitin within oomycete cell walls and the absence of ergosterol within oomycete cytoplasmic membranes. Additionally, oomycetes undergo sexual reproduction via oogamy.3
Oomycetes are typically found in warm stagnant water with Pythium sp. having been reported in multiple continents, including North and South America, southern parts of Asia, and Australia. Conversely, Lagenidium sp. has only been reported in southern regions of North America.2-4 Infection with oomycetes are rare in Wisconsin though this dog’s recent travel history from Texas was suspect. In dogs, Pythium insidiosum and Lagenidium giganteum are the most common etiologic agents of pythiosis and lagenidiosis, respectively.4,13 The life cycle of Pythium insidiosum first involves colonization of plants by Pythium insidiosum hyphae which then develop into zooporangia, eventually forming zoopores which mechanically break through the vesicle wall and are released into the stagnant water in which the plant resides.4,8 Zoopores are considered the infective stage and target damaged skin via direct contact or gastrointestinal mucosa if ingested through chemotaxis and its biflagellate motility.4,8 The zoospores encyst and form hyphal structures similar to the first life stage previously described. Exoantigens cause a T-helper 2 (Th2)-mediated immune response resulting in recruitment of eosinophils which then degranulate resulting in tissue damage.1,3,4,5,8 With time, the tissue damage will induce more pyogranulomatous inflammation as seen in this case.3-5 Little is known about the life cycle and pathogenesis of Lagenidium sp., though it is likely similar to Pythium sp..4 Some studies have shown that the life cycle and pathogenesis of Lagenidium sp. may involve mosquitoes.13
Pythium/Lagenidium sp. have been reported in dogs, cats and humans.4,11 In addition, Pythium sp. have been well-documented in horses and sporadically diagnosed in cattle, sheep, a Californian nestling white-faced ibis, bears, camels, and large cats (i.e. tigers and jaguars).1,3 In dogs and cats, Pythium sp. can cause cutaneous and more frequently, gastrointestinal tract lesions. Infections affecting horses and other documented species are typically limited to the cutis and subcutis.3,8 In contrast to Pythium sp., there are currently no reports documenting gastrointestinal lesions caused by Lagenidium sp.. However, Lagenidium sp. can cause cutaneous lesions and affect other tissues such as great vessels, sublumbar and inguinal lymph nodes, lung, pulmonary hilus, and cranial mediastinum.4
When possible, wide surgical excision and various antimicrobial agents and immunotherapeutic drugs have exhibited successful treatment in various species infected with Pythium/Lagenidium spp.3,4 Early detection of disease increases the probability of successful treatment; however, in many cases, there fails to be early detection and fatality occurs regularly.3,4 In addition, local recurrence is common and may occur at the surgical excision site or in a regional lymph node.4
Contributing Institution:
University of Wisconsin
School of Veterinary Medicine
Department of Pathobiological Sciences
2015 Linden Drive Madison, WI 53706
https://www.vetmed.wisc.edu/departments/pathobiological-sciences/
JPC Diagnosis:
Esophagus: Esophagitis, necrotizing and pyogranulomatous, chronic-active, multifocal to coalescing, severe, with numerous intrahistiocytic and extracellular hyphae.
JPC Comment:
This second case featured a tricky tissue identification for participants given the marked inflammation, and in this case, features of a somewhat “hidden” organiam were more readily recognized. The nature of the distal esophagus transitioning from a stratified squamous epithelium to columnar epithelium gave some members pause, though this is actually a normal histologic feature6 and not transitional epithelium (i.e. the urinary bladder).
Dr. Alves emphasized the chronic-active nature of this case. In section, there was a spectrum of inflammatory changes including coalescing areas of necrosis and discrete granulomas as well as maturing granulation tissue and fibrosis. These changes were largely within the serosal tunia, but did expand into the muscularis and submucosa, resulting in degeneration and necrosis of skeletal muscle and loss of esophageal glands in this particular section. GMS and PAS both outlined organisms well in section.
Conference participants then discussed the morphologic findings of the fungal hyphae in this case. We agree with the contributor that the major histologic features of the hyphae are consistent with Pythium/Lagenidium sp. and not with Cladosporium. Several conference participants raised the possibility of Conidiobolus/Basidiobolus sp. as another differential diagnosis. Associated hyphae are typically larger than oomycetes8 (approximately 5-12 µm and 6-20µm respectively) though have the same irregular branching and rare septations present.8 Though rare, gastrointestinal basidiobolomyocsis has been recorded both in the human11 and veterinary7 literature. In contrast, Conidiobolus is typically associated with respiratory disease in mammals.7 In histologic section, Basidiobolus would be expected to elicit a strong Th2-response similar to what the contributor describes for this case. However, the chronicity of the lesion may also influence whether it is primarily eosinophilic. In this case, the degree of fibrosis and formed granulomas overshadowed eosinophils.
References:
- Chitasombat MN, Larbcharoensub N, Chindamporn A, Krajaejun T. Clinicopathological features and outcomes of pythiosis. Int J Infect Dis. 2018;71:33-41.
- Chitasombat MN, Jongkhajornpong P, Lekhanont K, Krajaejun T. Recent update in diagnosis and treatment of human pythiosis. Peer J. 2020 Feb 20;8:e8555.
- Gaastra W, Lipman LJ, De Cock AW, Exel TK, Pegge RB, Scheurwater J, Vilela R, Mendoza L. Pythium insidiosum: an overview. Vet Microbiol. 2010;146(1-2):1-16.
- Grooters AM, Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet Clin Small Anim. 2003;33:695-720.
- Grooters AM, Hodgin EC, Bauer RW, Detrisac CJ, Znajda NR, Thomas RC. Clinicopathologic Findings Associated with Lagenidium sp. Infection in 6 Dogs: Initial Description of an Emerging Oomycosis. Vet Intern Med. 2003;17:637-646.
- Mann CV, Shorter RG. Structure of the canine esophagus and its sphincters. Journal of Surgical Research. 1964 April; 4(4):160-163.
- Marclay M, Langohr IM, Gaschen FP, et al. Colorectal basidiobolomycosis in a dog. J Vet Intern Med. 2020; 34: 2091–2095.
- Mendoza L, Hernandez F, Ajello L. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J Clin Microbiol. 1993 Nov;31(11):2967-73.
- Rodrigues Hoffmann A, Ramos MG, Walker RT, Stranahan LW. Hyphae, pseudohyphae, yeasts, spherules, spores, and more: A review on the morphology and pathology of fungal and oomycete infections in the skin of domestic animals. Veterinary Pathology. 2023;60(6):812-828.
- Romero A, Garcia J, Balestie S, Malfatto F, Vicentino A, Sallis ES, Schild AL, Dutra F. Equine pythiosis in the estern welands of Uruguay. Pesq Vet Bras. 2019;39(7):469-475.
- Velázquez-Jiménez Y, Hernández-Castro R, Romero-Romero L, Salas-Garrido CG, Martínez-Chavarría LC. Feline Phaeohyphomycotic Cerebellitis Caused by Cladosporium cladosporioides-complex: Case Report and Review of Literature. J Comp Path. 2019;170:78-85.
- Vikram HR, Smilack JD, Leighton JA, Crowell MD, De Petris G. Emergence of gastrointestinal basidiobolomycosis in the United States, with a review of worldwide cases. Clin Infect Dis. 2012 Jun;54(12):1685-91.
- Vilela R, Taylor JW, Walker ED, Mendoza L. Lagenidium giganteum Pathogenicity in Mammals. Emerg Infect Dis. 2015;21(2):290-297.