WSC 2022-2023:
Conference 16:
Case I:
Signalment:
11-year-old, intact male, snow leopard (Panthera uncia)
History:
The animal was diagnosed with COVID-19 via PCR and was doing well until an acute decline. Frank hemorrhage exuded from the nares, and blood was present on rectal.
Gross Pathology:
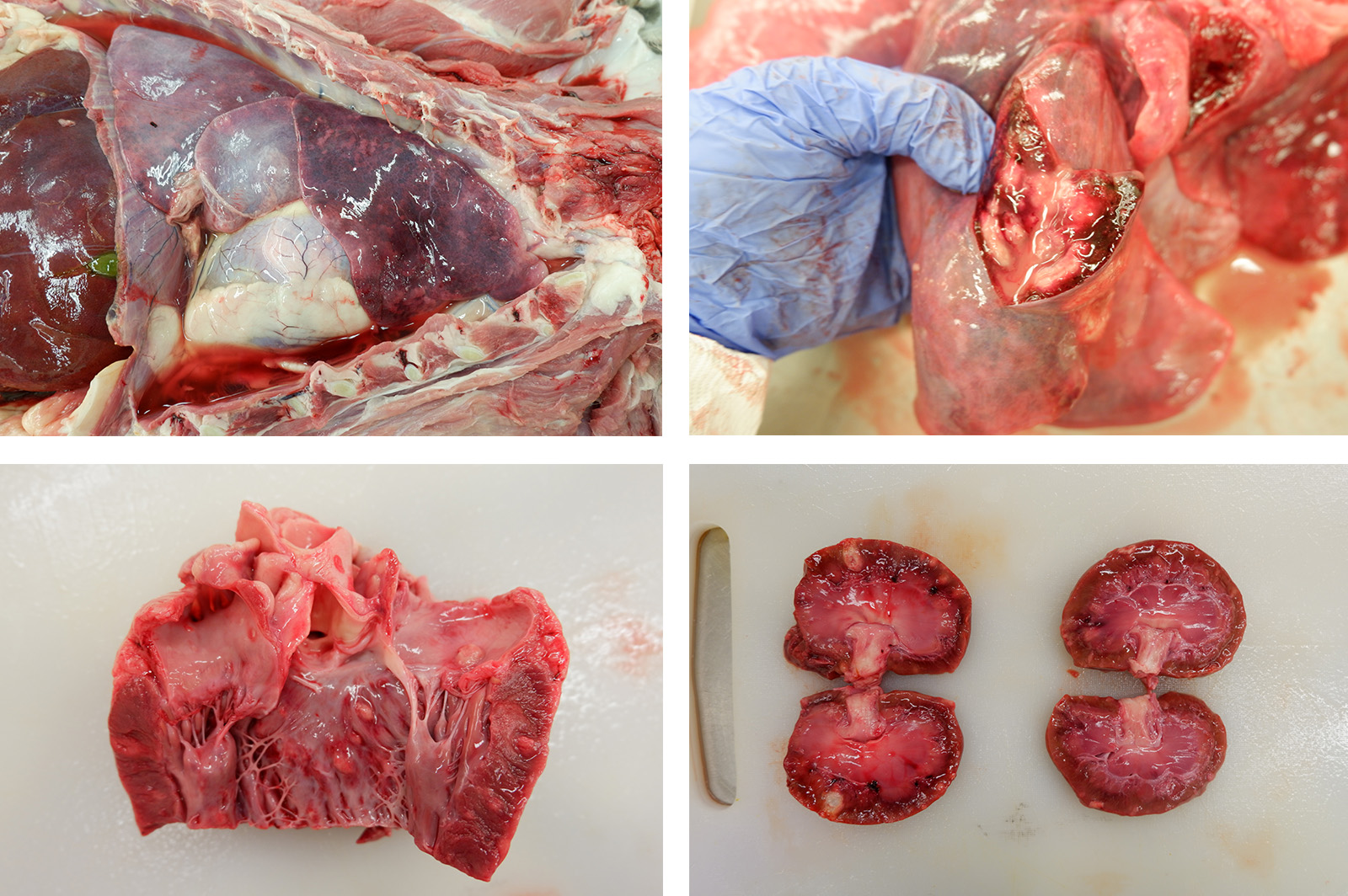
Gross Description: A moderate amount of dark red, dried blood was adhered to the fur around both nares. The thoracic cavity contained 164 mL of serosanguinous, semi-translucent fluid with few strands of fibrin. The lungs were red to dark red, wet, and edematous. Scattered throughout the lung were few tan to white mottled, nodular to multinodular masses, the largest of which was 4.0 cm x 3.2 cm x 2.7 cm. Similar nodules were present within the heart, bulging from the left atrioventricular endocardium and extending into the myocardium. The cortex and medulla of both kidneys were also expanded by numerous randomly scattered tan, bulging, masses like those within the lung and heart.
Laboratory Results:
- Thoracic radiographs: Bilateral alveolar pattern of the caudodorsal lung fields
- Covid-N rRT-PCR: Positive
- In situ hybridization with v-SARS-CoV-2-N-01 (N gene) probe of lung, nasal, and tracheal sections: Distinct punctate staining in rare sloughed epithelial cells within nasal passages, in cells within alveoli (macrophages and/or sloughed epithelial cells), and within rare pneumocytes lining alveoli. These findings confirm the presence of SARS-CoV-2 RNA within tissue sections, albeit at low levels.
4.18s rRNA PCR Sequencing of FFPE Lung tissue: 99.70% identity to Scedosporium apiospermum
Microscopic Description:
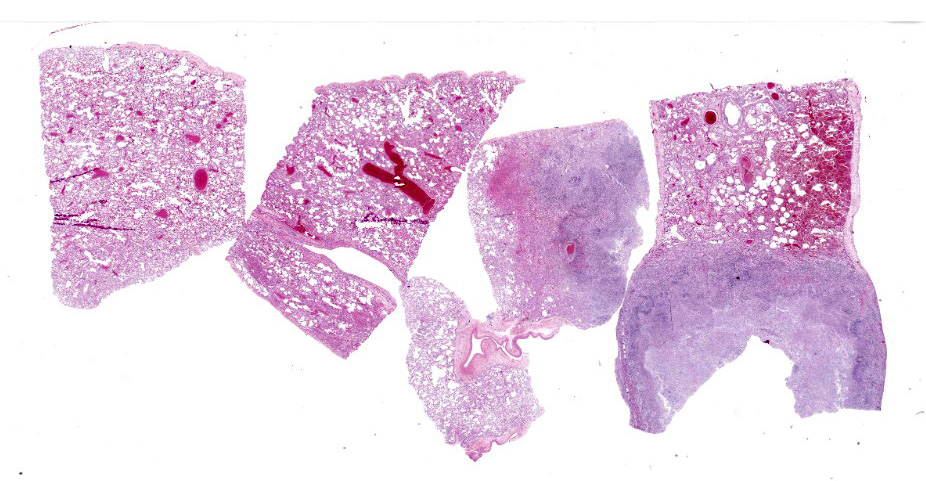
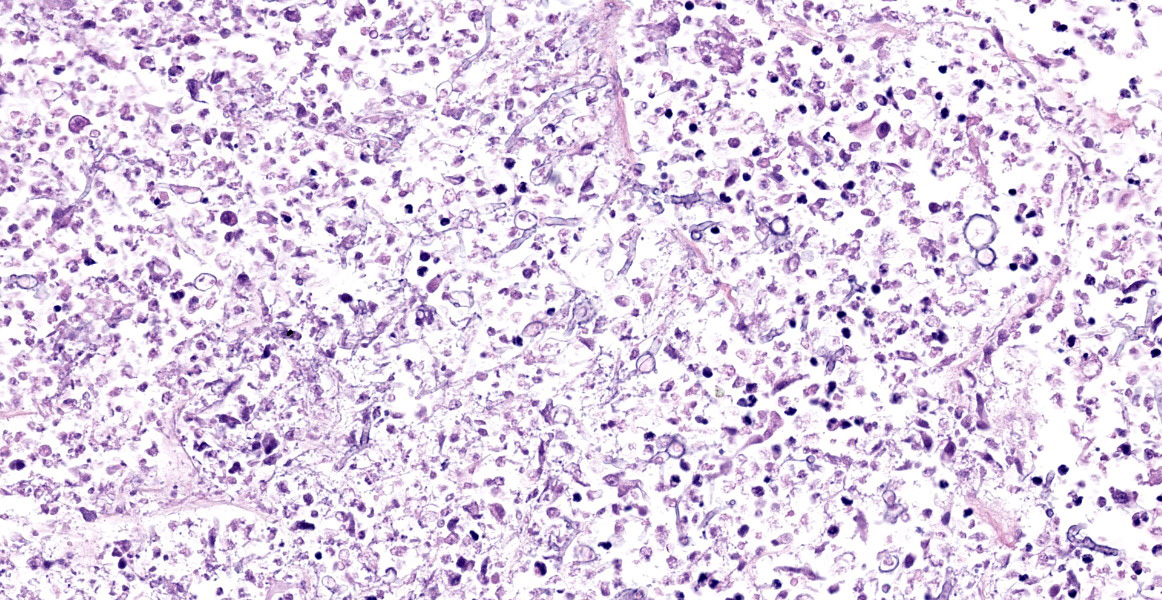
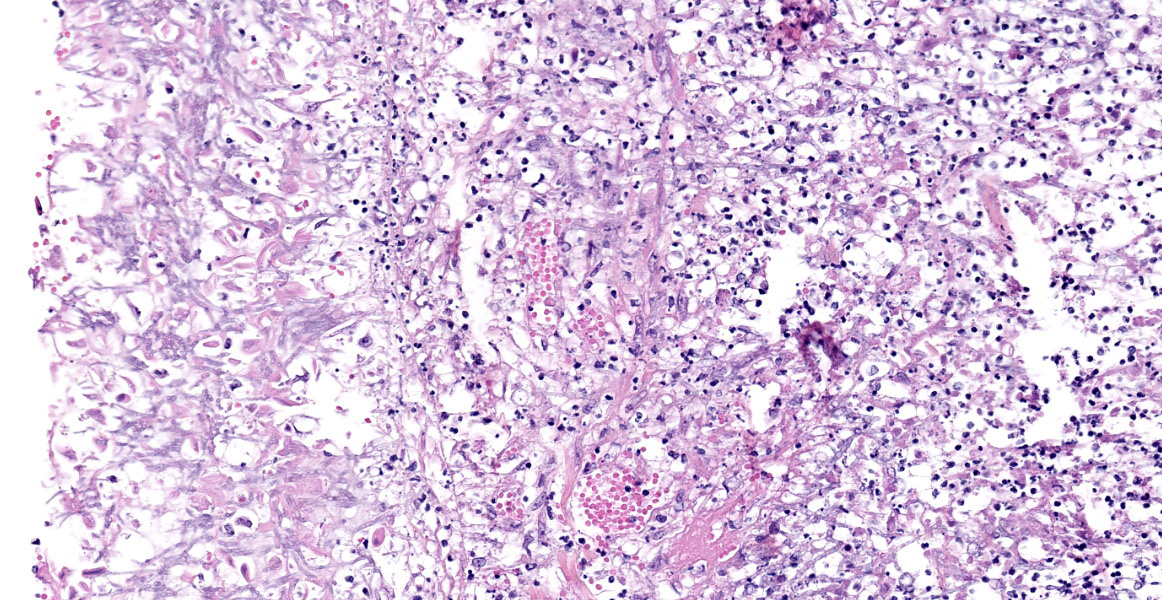
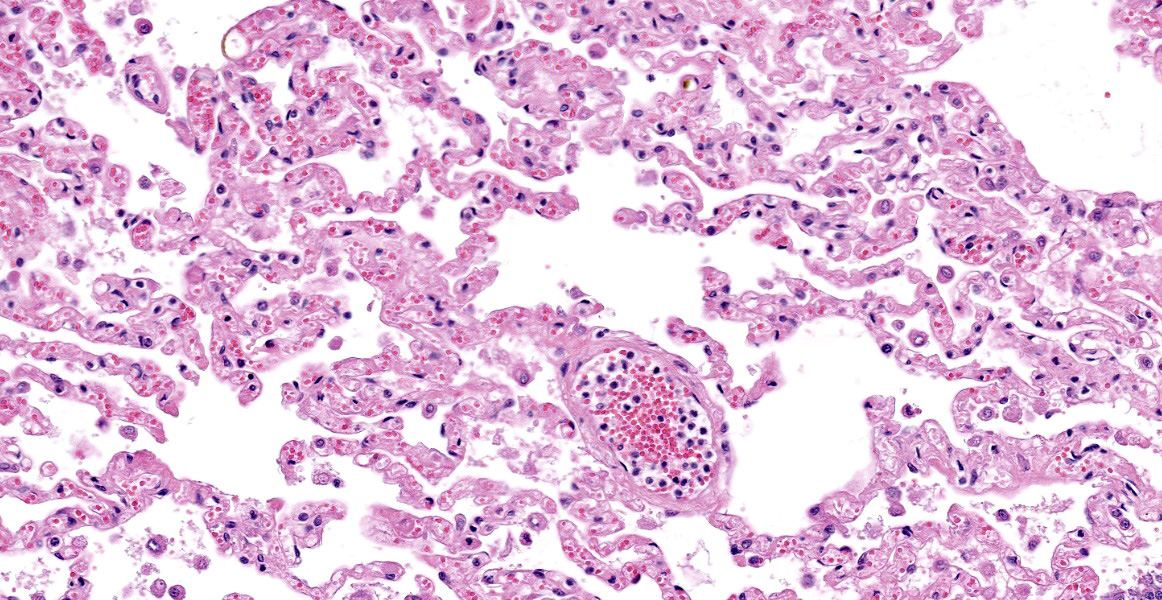
Lung: Scattered throughout the lung parenchyma are multiple large nodules composed of a core of eosinophilic and karyorrhectic cell debris mixed with abundant fibrin, some edema, and hemorrhage. Cores are surrounded by a dense rim of numerous necrotic neutrophils and epithelioid macrophages mixed with fewer lymphocytes and plasma cells. Throughout the necrotic cores and extending into the inflammatory rim are numerous fungal hyphae that are 3-6 µm wide with parallel walls, regular septations, dichotomous acute angle branching, and bulbous dilatations (presumptive ascomycete fungus). Throughout these nodules, septal vessels often contain thrombi composed of layers of lamellated fibrin. In many areas, septa are coagulatively necrotic, characterized by maintained architecture with diffuse hypereosinophilic and loss of nuclear detail. Rarely, necrotic debris is mineralized. Many alveolar septa are expanded by congestion, eosinophilic fibrillar material (fibrin), clear and colorless space (edema) and foamy macrophages; some septa contain smooth muscle hypertrophy and hyperplasia. Type 1 pneumocytes are often absent and replaced by deeply eosinophilic lamellar material (hyaline membranes). In some areas type 1 pneumocytes are replaced by cuboidal epithelium which segmentally lines alveolar spaces (type 2 pneumocyte hyperplasia). Alveolar spaces are frequently filled with variable combinations of foamy macrophages, eosinophilic proteinaceous material (edema), extravasated erythrocytes, fibrin, and neutrophils. Similar accumulations are present in many bronchioles and bronchi.
Other histopathology findings that are not included in the provided slide:
- Multifocal, chronic, pyogranulomatous necrotizing encephalitis, myocarditis, nephritis, and bilateral retinitis and choroiditis, with left eye scleritis and posterior uveitis, with intralesional hyphae
- Moderate, diffuse, chronic rhinitis with fibrosis
- Moderate, multifocal to coalescing, lymphoplasmacytic tracheitis with erosions and ulcerations
Contributor’s Morphologic Diagnoses:
- Lung: Marked, subacute interstitial pneumonia with type-2 pneumocyte hyperplasia and hyalin membrane formation, and marked hemorrhage and edema
- Lung: Marked, multifocal, chronic, pyogranulomatous necrotizing pneumonia with intralesional hyphae.
Contributor’s Comment:
Two main processes are identified in this animal, which contributed to death. The first is significant alveolar damage and interstitial inflammation accompanied by abundant pulmonary edema and hemorrhage, which is histologically compatible with SARS-CoV2 infection. The pathophysiologic mechanisms of COVID-19 are incompletely understood, however direct effects are primarily limited to the lung, while systemic disease occurs via secondary effects.2 Within humans, the histopathologic lesions associated with severe COVID-19 are consistent with Diffuse Alveolar Damage (DAD) secondary to Acute Respiratory Distress Syndrome (ARDS). DAD is not specific for COVID-19, and can be induced by various causes, including mechanical ventilation, various pulmonary infections, thermal injury, toxic gas exposure, and septicemia.3
The exact appearance of DAD will vary depending on the chronicity. An acute, exudative phase occurs during the first week after pulmonary injury, and is characterized by congested alveolar septa, and alveoli filled with copious protein-rich edema and fibrin. Inflammation is generally low unless DAD is the result of previous pneumonia. The formation of hyalin membranes—aggregates of fibrin, other serum proteins, and cell debris which line alveoli and alveolar ducts, impeding the junction between the airspace and the septum—is an important characteristic histologic finding.3,10 Over time, DAD will progress into an organizing or proliferative phase, characterized by interstitial proliferation of fibroblasts and myofibroblasts, accompanied by type 2 pneumocyte hyperplasia and squamous metaplasia.2 Microvascular thrombi are also frequently seen with COVID-19, however it is speculated that this lesion is more associated with ARDS than COVID-19 specifically.2
COVID-19 has been seen within multiple animal species, including domestic cats, domestic dogs, tigers, lions, gorillas and snow leopards (USDA Confirmed Cases of SARS-CoV-2 in animals in the United States). A recent study has shown that domestic cats (Felis catus) can be utilized as an infection model for COVID-19, displaying similar clinical and histopathologic as in humans.13 COVID-19 viral infection and replication within a host depends on the presence and distribution of angiotensin-converting enzyme 2 (ACE2) receptors.2 The ACE2 receptor within cats is similar in structure and distribution to humans, which may contribute to the similarities in clinical and histologic findings.13 It has not been studied if these same principles apply to large felids such as snow leopards. Within this case, interstitial and alveolar changes within the lung are consistent with a sub-acute DAD response transitioning between the exudative phase and proliferative phase. While there are multiple differentials for a DAD response within this animal, in situ hybridization confirms COVID-19 infection within the epithelial cells of the nasal passages and rare pneumocytes of the alveoli.
The second process in this case consists of severe pyogranulomatous and necrotizing lesions throughout multiple organs, including the lung, brain, heart, kidneys, and the choroid and retina of both eyes. Sequencing of the fungal organisms within these lesions identifies it as Scedosporium apiospermum, an opportunistic filamentous fungus found worldwide.6 Once considered the asexual form of Pseudoallescheria boydii, recent taxonomic changes upon introduction of molecular phylogenetics places S. apiospermum within the Scedosporium genus, where it is the most pathogenic of the included species.4,14 S. apiospermum is of increasing importance in human medicine, where it can cause severe systemic infections within immunocompromised and immunocompetent patients. Rare cases of both localized and disseminated infections have been reported in various animal species, including the dog, cat, horse, and a stranded northern elephant seal.1,4,8,9
Diagnosis of a Scedosporium infection is difficult due to clinical and histopathologic similarities to Aspergillus, Fusarium, and other hyalin hyphomycetes.7 Subtle histologic differences between Scedosporium and Aspergillus do exist, including slightly more irregular branching within Scedosporium vs the more regular and dichotomous branching pattern of Aspergillus. Additionally, Scedosporium will commonly have terminal or intercalary, globose chalamydospores, thick-walled structures up to 20um in diameter which can be confused with yeasts.7 These differences are subtle, and further testing such as in situ hybridization, culture, or molecular sequencing are important for definitive diagnosis. It is likely that Scedosporium is underdiagnosed in veterinary medicine due to its similarities to other fungal species.4 Definitive diagnosis of Scedosporium is important, due to differences in antifungal resistance profile from Aspergillus.6
In this case, it is speculated that the primary COVID-19 infection led to immunocompromise of the animal, and systemic opportunistic infection by Scedosporium apiospermum, demonstrating both the importance of COVID-19 within the snow leopard species, and the growing importance of Scedosporium apiospermum within veterinary medicine.
Contributing Institution:
University of Illinois at Urbana-Champaign, Veterinary Diagnostic Laboratory
JPC Diagnosis:
- Lung: Pneumonia, interstitial, necrotizing, subacute, diffuse, marked, with septal thrombosis, hemorrhage, and type II pneumocyte hyperplasia.
- Lung: Pleuropneumonia, pyogranulomatous and necrotizing, multifocal, severe, with innumerable fungal hyphae.
JPC Comment:
A review of recent literature demonstrates that experimental severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection in domestic cats can produce a spectrum of clinical disease and histologic lesions. As the contributor describes, one possible outcome is diffuse alveolar damage, as described by Rudd et al in a 2021 Viruses article.13 In Rudd’s study, the virus was inoculated directly into the trachea of 12 nine-month-old specific pathogen free (SPF) cats; six cats were used as controls.13 Inoculated cats developed clinical signs of illness, including lethargy, fever, and dyspnea, and histologic evidence of diffuse alveolar damage.13
In a prior study, Gaudreault et al inoculated 6 five-month-old SPF cats with a slightly larger volume of the same strain of virus, but through nasal and oral routes.5 These animals remained asymptomatic but transmitted the virus to co-housed, uninfected animals.5 Histopathologic findings were minimal to moderate and localized to conducting airways, with lymphocytic and neutrophilic inflammation of the tracheobronchial seromucous glands which resolved by 21 days post challenge.5
Finally, a more recent study published by Patania et al in Vet Pathol described a different pattern of interstitial pneumonia with occlusive bronchiolitis in experimentally infected cats.12 Ten SPF cats, ages 19 to 24 week, were inoculated by a different viral strain through intraocular, intranasal, tracheal, and oral routes.12 No control cats were used. The cats remained asymptomatic, but on histology, there was patchy interstitial pneumonia, histiocytic bronchiolitis, and persistent interstitial thickening. Most of the cats lacked diffuse alveolar damage.12 Bronchiolar lumens were often occluded by adherent plugs of epithelioid macrophages.12 Early in the disease, interstitial and alveolar septal thickening was partially attributed to a mixed inflammatory infiltrate composed of B lymphocytes, T lymphocytes, and macrophages.12 Once the inflammation resolved, interstitial thickening persisted and was attributed to endothelial hyperplasia, proliferative and disorganized capillaries, and individualized type II pneumocyte hyperplasia only appreciable with cytokeratin IHC; fibrosis was not a feature in thickened septae.12 One of the three cats evaluated at 28 days post-challenge had more severe changes, including atelectasis, vasculitis, and inflammatory exudates and fibrin within alveoli.12
The differences in the results of these studies illustrate the wide range of lesions which may be induced by experimental SARS-CoV2 infections in cats, and differences in study design (routes of inoculation, challenge dose, viral strain used) and individual host responses may account for these results.
Invasive fungal diseases (IFD) secondary to SARS-CoV-2 infection occur with relative frequency in hospitalized human patients.11 Up to 7.6% of hospitalized COVID-19 experience pulmonary aspergillosis due to Apsergillus fumigatus, and this coinfection has a mortality rate of 56%.11 Other documented coinfections include candidiaisis and mucormycosis, most commonly due to Rhizopus.11 There are multiple possible reasons why COVID-19 patients are more susceptible to fungal infections, including tissue damage, immune dysfunction, and individual host factors or preexisting conditions. Viral infection impairs mucocilliary clearance and causes local tissue damage, exposing hidden host receptors permissive to fungal invasion.11 Viral induced lymphopenia, depletion and dysfunction of dendritic cells, and local hypoxia attenuate the immune response to fungal invasion; this can be exacerbated by the use of immunsuppressive agents prescribed for controlling the overwhelming inflammatory response.11 Chronic viral infection leads to decreased numbers of CD8+ T cells and NK cells, and remaining cells may demonstrate an ineffective and immune-exhausted phenotype (expressing PD1 and NKG2A, respectively) which create risk factors for IFB.11 COVID-19 may also cause impaired fungicidal activity by neutrophils, leading to impaired innate immunity.11
References:
- Berzina I, Trumble NS, Novicki T, et al. Subconjunctival mycetoma caused by Scedosporium apiospermum infection in a horse. Vet Clin Pathol. 2011; 40(1):84-88.
- Caramashi S, Kapp ME, Miller SE, et al. Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review. Pathol. 2021; 34:1614-1633.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th St. Louis, MO: Elsevier; 2016.
- Di Teodoro G, Averaimo D, Primavera Miria, et al. Disseminated Scedosporium apiospermum infection in a Maremmano-Abruzzese sheepdog. BMC Vet Res. 2020: 16(2):372.
- Gaudreault NN, Trujillo JD, Carossino M. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. 2020; 9(1): 2322-2332.
- Goldman C, Akiyama MJ, Torres J, et al. Scedosporium apiospermum infections and the role of combination therapy and GM-CSF: A case report and review of the literature. Mycol Case Rep. 2016; 11:40-43.
- Guarro J, Kantarcioglu AS, Horre R, et al. Scedosporium apiospermum: a changing clinical spectrum of a therapy-refractory opportunist. Mycol. 2006; 44(4):295-327.
- Haulena M, Buckles E, Gulland FMD, et al. Systemic mycosis caused by Scedosporium apiospermum in a stranded northern elephant seal (Mirounga angustirostris) undergoing rehabilitation. Zoo Wildl Med. 2002; 33(2):166-171.
- Leperlier D, Vallefuoco R, Laloy E, et al. Fungal rhinosinusitis caused by Scedosporium apiospermum in a cat. J Feline Med Surg. 2010; 12:697-971.
- Montero-Fernandez MA, Pardo-Garcia R. Histopathology features of the lung in COVID-19 patients. Diagn Histopathol. 2021; 27(3):123-127.
- Morton CO, Griffiths JS, Loeffler J, Orr S, White PL. Defective antifungal immunity in patients with COVID-19. Front Immunol. 2022; 13:1-11.
- Patania OM, Chiba S, Halfmann PJ, et al. Pulmonary lesions induced by SARS-CoV-2 infection in domestic cats. Vet Pathol. 2022; 59(4): 696-706.
- Rudd JM, Selvan MT, Cowan S, et al. Clinical and histopathologic features of a feline SARS-CoV-2 infection model are analogous to acute COVID-19 in humans. 2021;13(8):1550.
- Taylor A, Talbot J, Bennett P, et al. Disseminated Scedosporium prolificans infection in a Labrador retriever with immune mediated haemolytic anaemia. Med Mycol Case Rep. 2014; 6:66-69.