Signalment:
Gross Description:
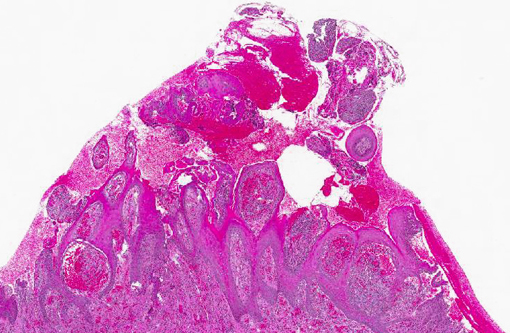
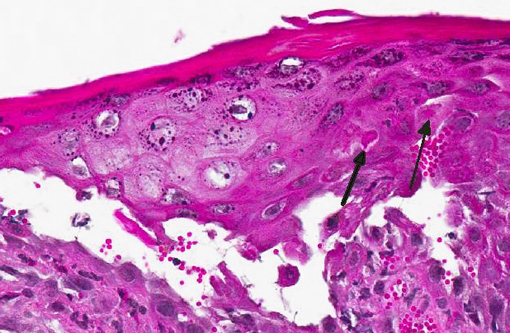
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Gross lesions are characterized by multifocal to coalescing proliferative and ulcerative dermatitis which is localized to mucocutaneous junctions, particularly around the mouth and nares.(1,3,5,9,14) Histologic lesions are characterized by ballooning degeneration, exuberant epidermal hyperplasia with intracorneal pustules, and eosinophilic intracytoplasmic inclusion bodies within the stratum spinosum that are only briefly detectable at the vesicular stage.(1,3,4,8,9,17) There are frequently superimposed bacterial infections in the affected skin.(13)
Sheep previously exposed to orf virus can be repeatedly infected although the severity of the lesions and time to resolution diminishes with each subsequent infection.(1,6,8,13) This indicates that the host immune response can control the severity of disease but cannot prevent reinfection.(6) The reason is due, at least in part, to the presence of at least five immunomodulatory proteins expressed by orf virus that subvert or suppress elements of the host immune and inflammatory response, including:(2,5-8,11)
(1) Orf virus interferon resistance protein (OVIFNR): Host cells generally attempt to stop viral replication through interferon-induced activation of the double stranded RNA-dependent kinase (PKR) pathway.(5,7,8) In the presence of viral double-stranded RNA, the PKR pathway is induced by interferon and binds to viral dsRNA which inhibits virus (and host cell) protein translation, effectively blocking virus replication.(5-8) The orf virus encodes a protein, OVIFNR, which is a homologue of vaccinia virus E3L gene product, competitively binds to dsRNA and disallows activation of PKR, which ultimately promotes a permissive state for viral replication, leading to sustained protein synthesis and completion of the virus life cycle.(5-8) In other words, the OVIFNR protein prevents activation of PKR and protects the virus from the antiviral effects of interferons, allowing viral replication.(5-8)
(2) Granulocyte/macrophage colony-stimulating factor (GM-CSF) inhibitory factor (GIF): GIF binds to and inhibits the biological activity of the cytokines GMCSF and interleukin2 (IL-2).(2,5-7) In non-hematopoietic tissues, GMCSF is involved in the recruitment and activation of macrophages and neutrophils.(6,8) GMCSF also supports the recruitment and antigen-presenting function of dendritic cells (DC).(6,8) IL2 is required for T cell proliferation and activation, for example, the augmentation of T cell IF gamma production and to expand CTL and NK cell populations.(6) Inhibition of these two important cytokines significantly impedes the development of both innate and acquired immune responses.
(3) Orf viral interleukin 10 (ORFV-IL-10): The initiation of an acquired immune response to a virus requires that antigen be taken up by DC at the periphery, and then processed and transported to lymphoid tissue to be presented to T cells.(7,11) For this to occur, DC must first be activated by signals transmitted through the interaction of viral products with pattern recognition receptors expressed on the surface of the DC.(11) DC therefore form the vital bridge between the innate and acquired arms of the immune response.(11) IL-10 is an immunoregulatory cytokine that inhibits both innate and adaptive immune responses and is produced by macrophages and dendritic cells in response to microbial products.(17) It is produced by multiple T cell subsets, including all three populations of helper T cells (Th1, Th2, and Th17).17 IL10 is able to down regulate MHC class II and co-stimulatory molecule expression on DC and macrophages, thus decreasing antigen presentation.(7,17) Orf virus-encoded interleukin-10 (ORFVIL10), a homologue of IL-10, has specifically been shown to suppress the migration of Langerhans cells (LC) and inhibit DC function in addition to suppressing inflammation and the innate immune response.(11) ORFV-IL-10 has the capacity to impair the initiation of an acquired immune response and hence inhibit the generation of immunological memory necessary for immunity of subsequent exposure.(11) ORFV-IL-10 may be capable of inhibiting the activation of memory cells recruited to infected skin following reinfection, as well as having the potential to inhibit the activation of na+â-»ve T cells that would otherwise be brought about by activated LC trafficking to the draining lymphoid tissue.(11) The implications of this are that on repeated cycles of infection, the virus can replicate, shed, and be transmitted to other hosts before elimination by the acquired immune response.(11)
(4) Orf virus chemokine-binding protein (CBP): Orf virus produces a novel CBP that binds CC chemokines such as monocyte chemotactic protein-1, macrophage inflammatory protein-1-alpha and RANTES (regulated upon activation normal T cell expressed and secreted) that control monocyte/macrophage and T cell recruitment to sites of infection.(5) Orf virus CBP also binds lymphotactin, a C chemokine that recruits T cells, B cells and neutrophils through the XCR1 receptor.(5) This provides further evidence that orf virus has evolved to inhibit important cellular elements of an anti-viral immune response.(5)
(5) Orf virus vascular endothelial growth factor (VEGF): Orf virus VEGF functions in the same way as cellular VEGFs, causing vascular proliferation and angiogenesis.(5,6,8,16) Orf virus lesions in sheep are characterized by vascular proliferation and dilation as well as epidermal proliferation.(5,8,16) The induction of epidermal proliferation by orf virus is probably important in supplying an abundance of target epidermal cells for virus replication.(5,8,16) Additionally, host cell VEGF has the ability to inhibit the development and maturation of dendritic cells.(5,8) If viral VEGF also has this ability, it would benefit the virus because of the important role that dendritic cells play in the generation and maintenance of immune responses.(5)
JPC Diagnosis:
Conference Comment:
References:
2. Deane D, McInnes CJ, Percival A, et al. Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2. J Virol. 2000;74(3):1313-1320.
3. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:326-327.
4. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:664-666.
5. Haig DM. Orf virus infection and host immunity. Curr Opin Infect Dis. 2006;19(2):127-131.
6. Haig DM. Subversion and piracy: DNA viruses and immune evasion. Res Vet Sci. 2001;70(3):205-219.
7. Haig DM, McInnes CJ, Thomson J, et al. The orf virus OV20.0L gene product is involved in interferon resistance and inhibits an interferon-inducible, double-stranded RNA-dependent kinase. Immunology. 1998;93(3):335-340.
8. Haig DM, Mercer AA. Orf. Vet Res. 1998;29(3-4):311-326.
9. Hargis AM, Ginn PE. The integument. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:1023.
10. Klein J, Tryland M. Characterization of parapoxviruses isolated from Norwegian semi-domesticated reindeer (Rangifer tarandus tarandus). Virology Journal. 2005;2:79-89.
11. Lateef Z, Fleming S, Halliday G, et al. Orf virus-encoded interleukin-10 inhibits maturation, antigen presentation and migration of murine dendritic cells. J Gen Virol. 2003;84(Pt 5):1101-1109.
12. McInnes CJ, Wood AR, Thomas K, et al. Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J Gen Virol. 2006;87(8):2115-2125.
13. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Poxviridae. In: Veterinary Virology. 3rd ed. San Diego, CA: Academic Press; 1999:289-291.
14. Navarre CB, Lowder MQ, Pugh DG. Oral-esophageal diseases. In: Pugh DG, ed. 1st ed. Sheep and Goat Medicine. Philadelphia, PA: W.B. Saunders Company; 2002:66-67.
15. Roess AA, Levine RS, Barth L, et al. Sealpox virus in marine mammal rehabilitation facilities, North America, 2007-2009. Emerg Infect Dis. 2011;17(12):2203-2208.
16. Savory LJ, Stacker SA, Fleming SB, et al. Viral vascular endothelial growth factor plays a critical role in orf virus infection. J Virol. 2000;74(22):10699-10706.
17. Tizard IR. Regulation of adaptive immunity. In: Veterinary Immunology. 9th ed. St. Louis MO: Elsevier; 2013:217.
18. Zachary JF. Mechanisms of microbial infections. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:210.