Wednesday Slide Conference, Conference 17, Case 4
Signalment:
8 week old, female intact, C.B-17 scid mouse (Mus musculus).
History:
This mouse was part of research project to study the role B. burgdorferi virulence factors in the pathogenesis of Lyme Borreliosis. The mouse was inoculated on the lumbosacral region via SQ with Borrelia burgdorferi B31- A3 strain and was euthanized 4 weeks post-challenge.
Gross Pathology:
Bilaterally, the tibotarsal joints were markedly thickened and swollen on the cranial aspect.
Laboratory Results:
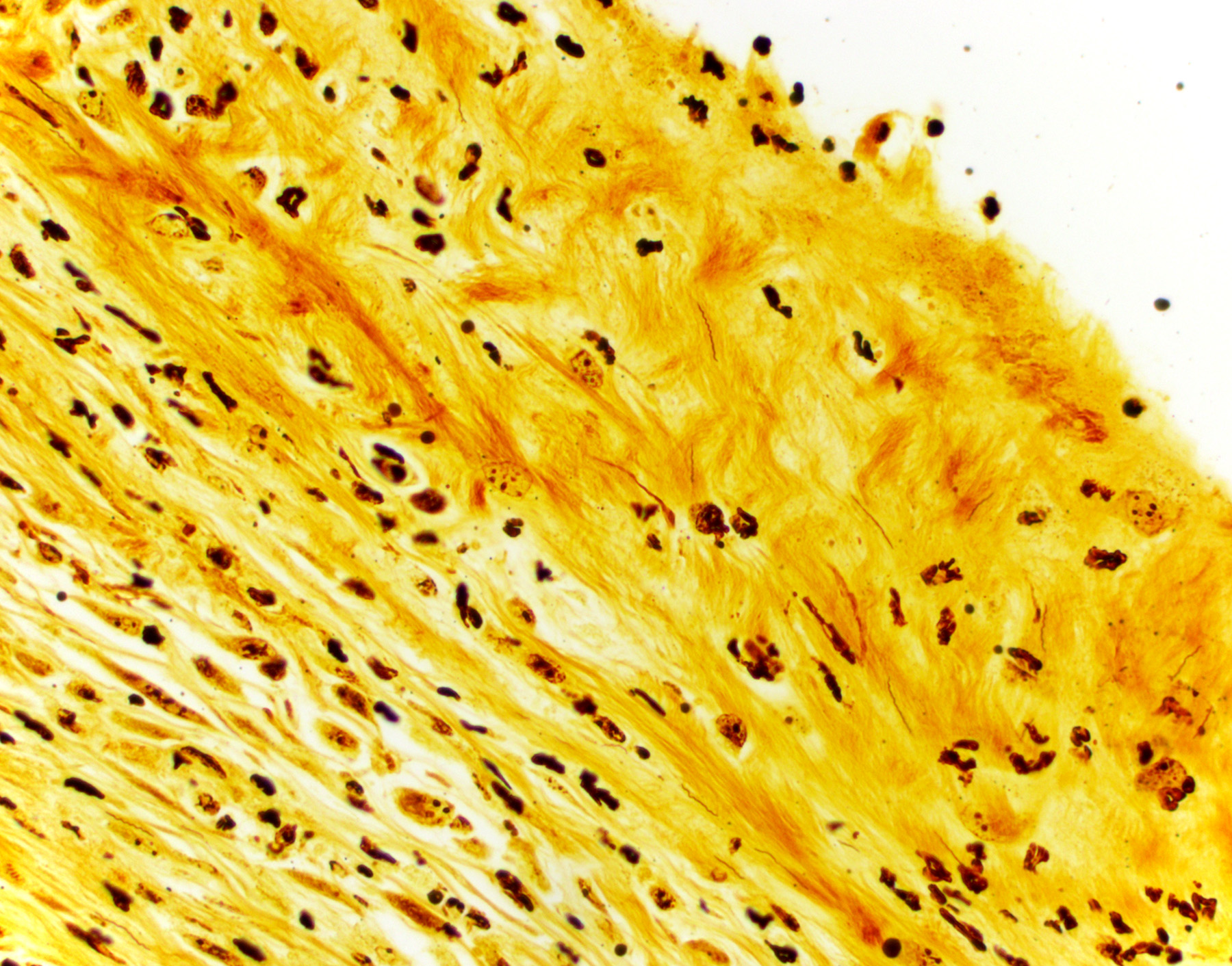
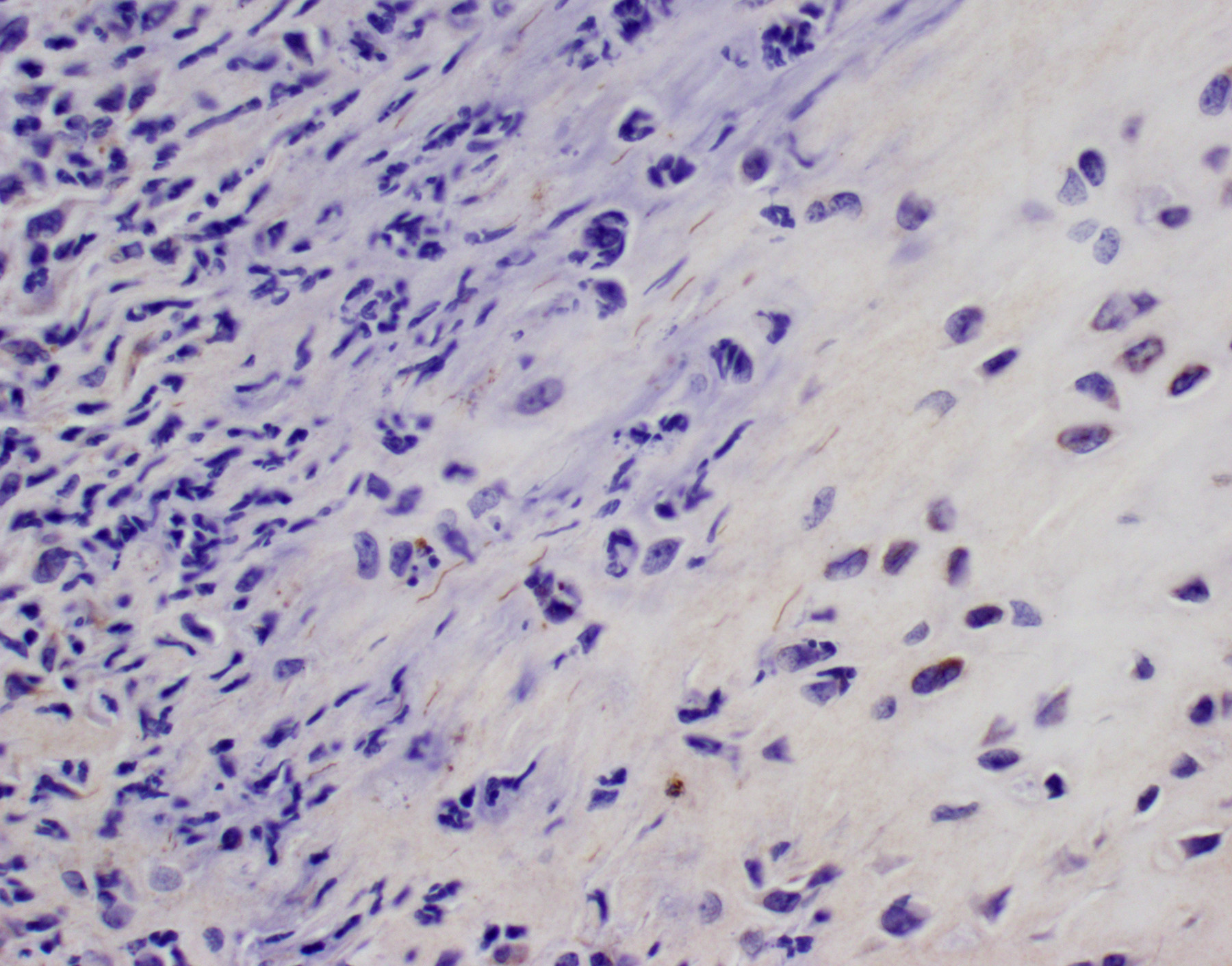
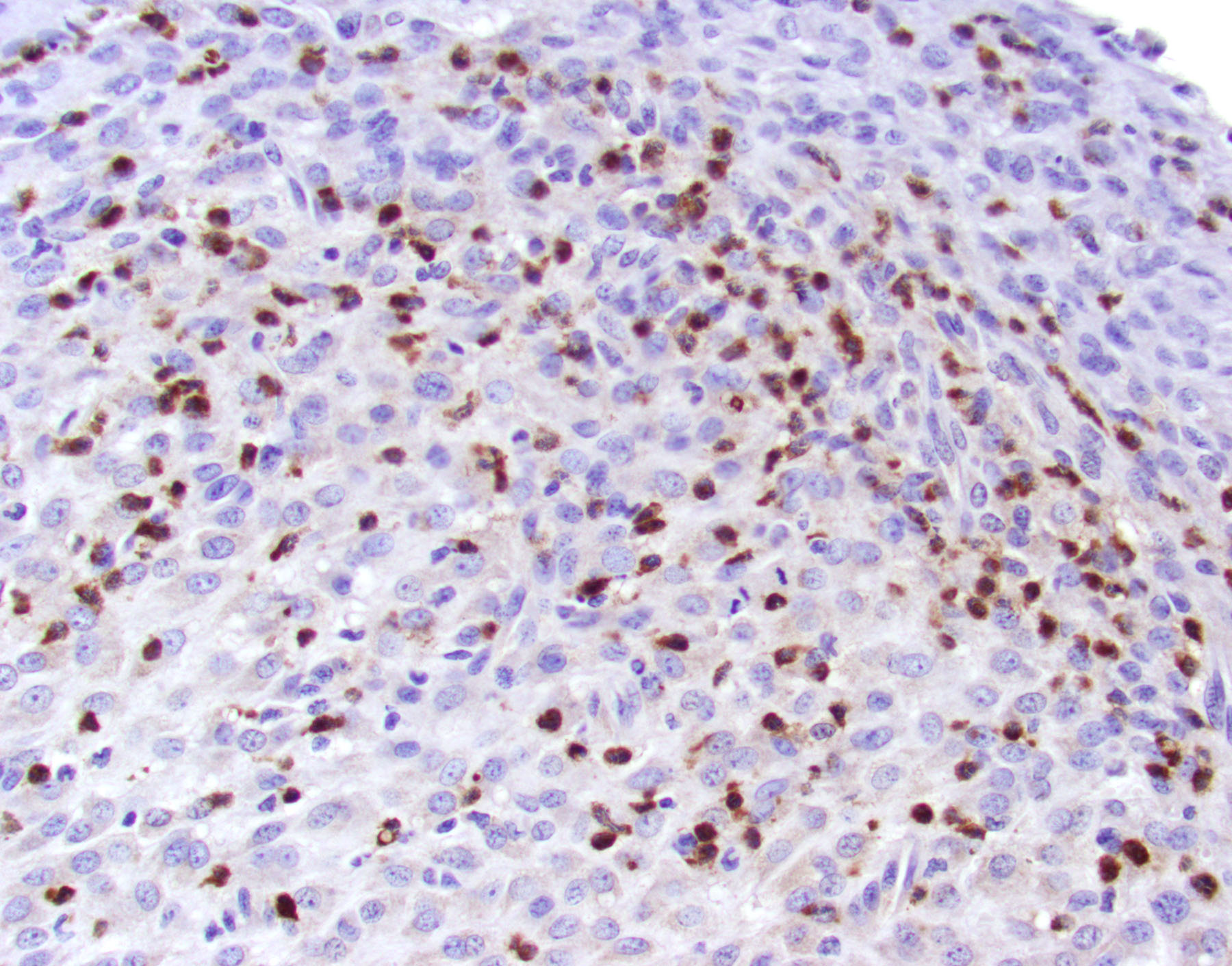
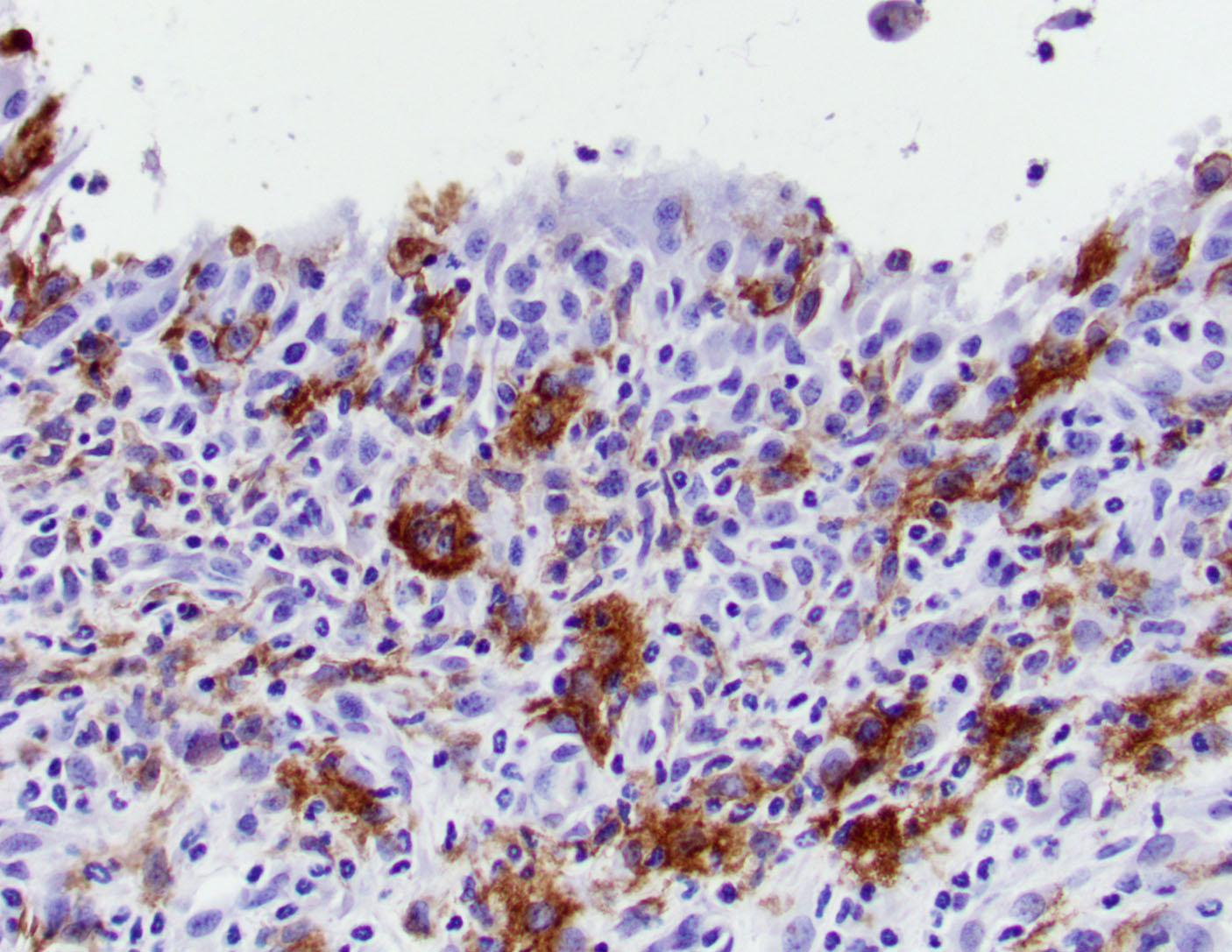
BSK-II culture media was positive for B. burgdorferi. Spirochetes were isolated from a section of the tibiotarsal joint. White blood cell counts demonstrated a neutrophilia (5.57, ref 0.43 – 3.58 K/uL). Spirochetes were demonstrated in areas of tenosynovitis by immunohistochemistry and Warthin-Starry stain in the tibiotarsal joints.
Microscopic Description:
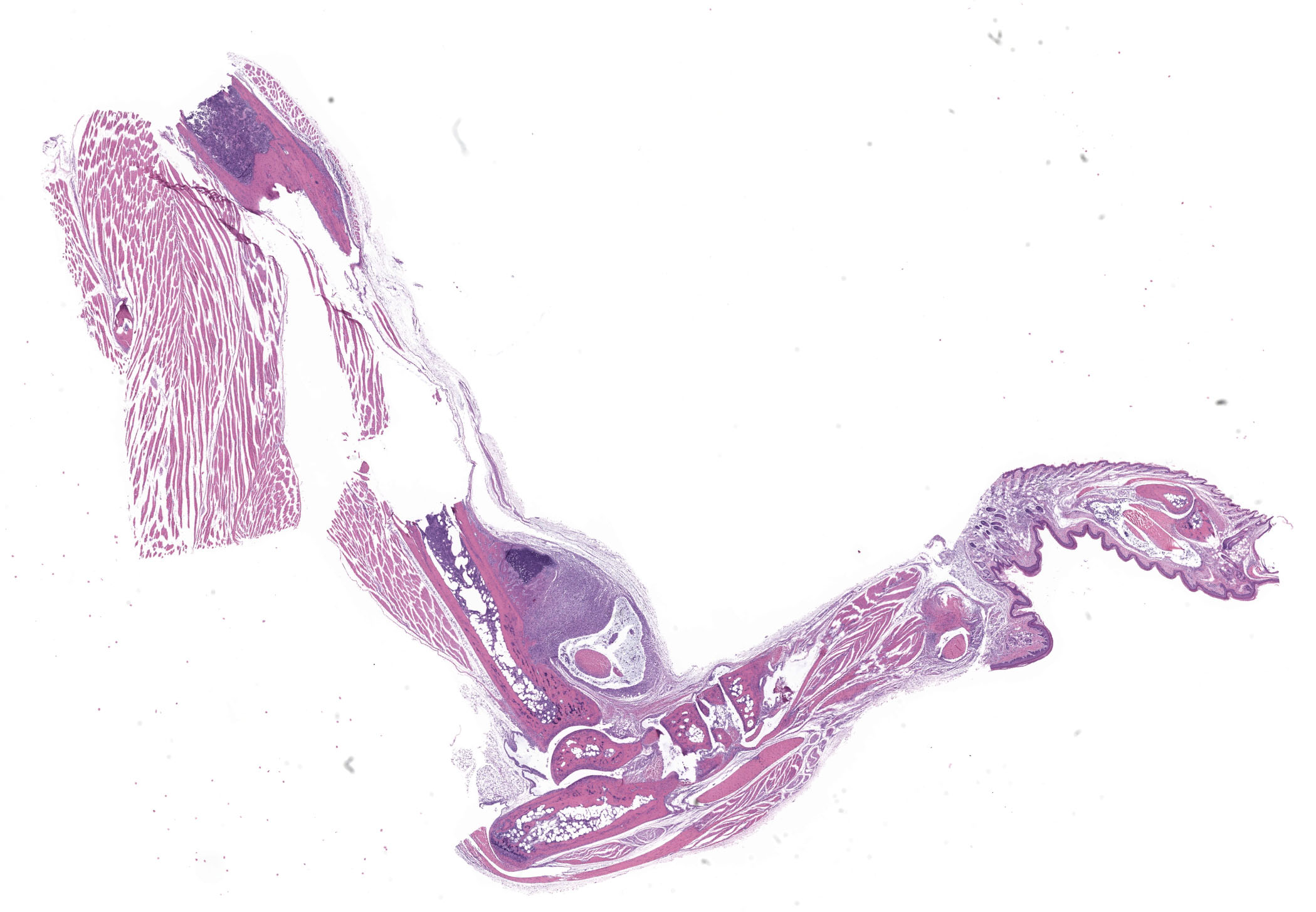
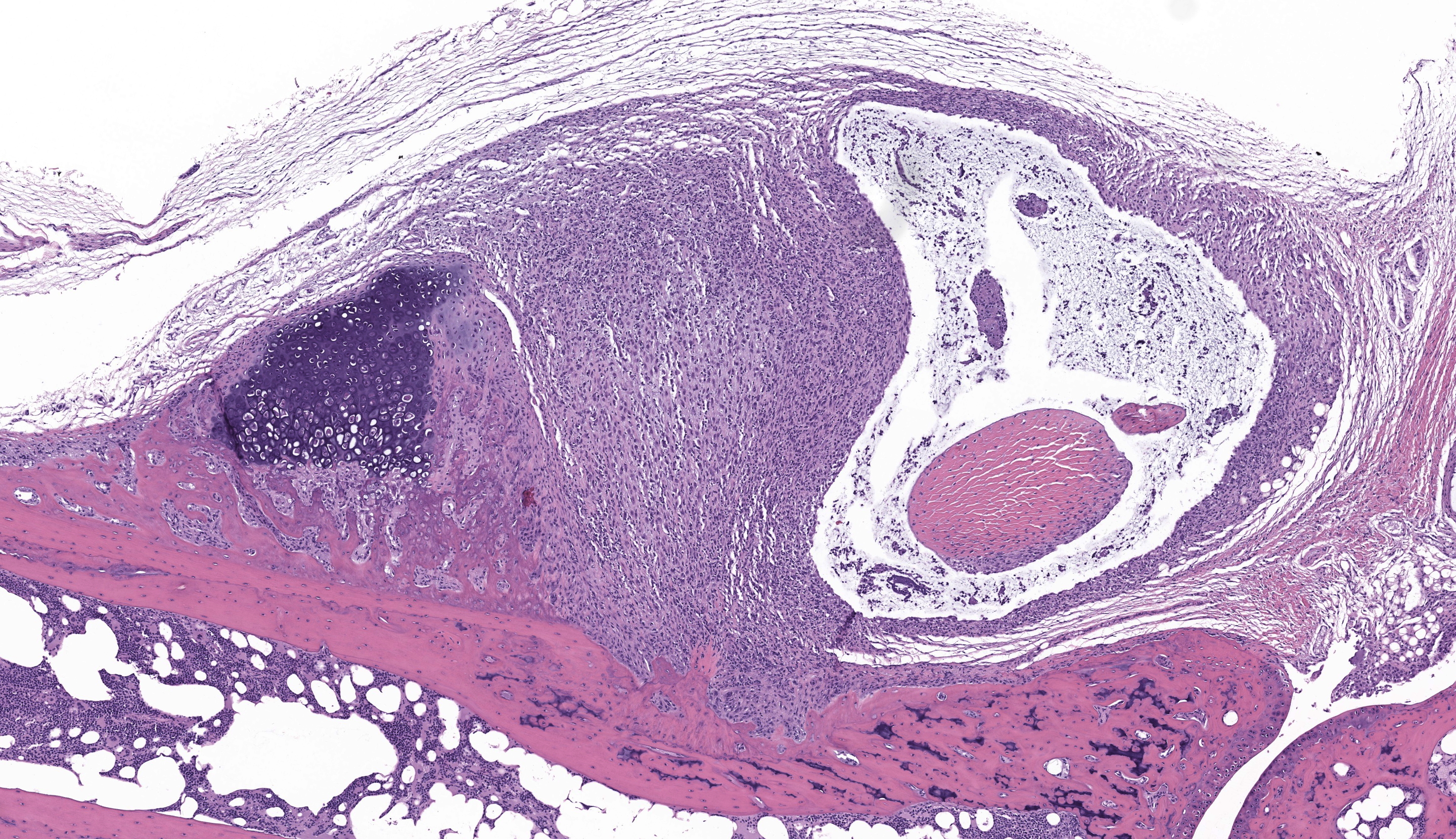
Tibiotarsal joint. Arising from antero-distal tibial metaphysis to the antero-proximal aspect of the tarsal bones is a well-demarcated, densely cellular, thickened and inflamed synovium, regionally admixed with an irregular trabecula of woven bone. The synovium is markedly hyperplastic by numerous, variably sized, spindle to spindloid synovial cells closely packed to each other and is lined by a discontinuous, single layer of plump synoviocytes. The synovium is diffusely infiltrated by large numbers of neutrophils and moderate numbers of macrophages. The synovial space and tendon sheaths are infiltrated by moderate numbers of neutrophils and fewer macrophages mixed with eosinophilic proteinaceous fluid, fibrin, sloughed cells, necrotic cells, and karyorrhectic debris. The periosteum along antero-distal tibial metaphysis and epiphysis is expanded by an irregular intertwined trabecula of woven bone that is incompletely mineralized (periosteal remodeling) and contains numerous osteocytes. The immature trabecula is lined by osteoblasts, entraps mesenchymal stromal cells, and is regionally bounded by organized island of cartilage undergoing endochondral ossification (bone formation). The edges of the woven bone are irregular and scalloped and often lined by osteoblasts and fewer osteoclasts within Howship’s lacunae (remodeling). Within the inflamed synovium a focal subperiosteal trabecula, arranged perpendicularly to zones of bone remodeling, is composed of woven bone at different stages of mineralization. The synovium and adjacent tendon sheaths in the remaining proximal tarsal bones and plantar aspect of the tibiotarsal joint is mildly hyperplastic and infiltrated by low numbers of macrophages. In these areas synovial spaces contain proteinaceous fluid mixed with individual sloughed synovial cells and rare karyorrhectic debris.
Contributor’s Morphologic Diagnosis:
Tibiotarsal joint: Tenosynovitis, neutrophilic and histiocytic, with synovial hyperplasia and hypertorphy, periosteal bone formation and remodeling, regional, marked, chronic.
Contributor’s Comment:
The C.B-17 scid (SCID) mouse presented in this submission was part of a study to determine the pathogenicity and tropism of Borrelia burgdorferi strains deficient in different virulence factors. The mouse was part of a control group of SCID mice that were inoculated with a high dose of B. burgdorferi B31-A3 (wild-type) strain. C.B-17 scid (SCID) mice are homozygous for the Prkdcscid mutation resulting in severe combined immunodeficiency.7 This mouse strain lacks functional lymphocytes (both T and B cells) because of impaired VDJ rearrangement.7 SCID mice exhibited the following features: marked lymphopenia, agammaglobulinemia, and high susceptibility to opportunistic infections.7,11
The use of laboratory mice has provided valuable insights into the immune response and pathogenesis of Lyme arthritis and carditis. Mice infected with Borrelia burgdorferi (Bb) can also develop myositis, arteritis/vasculitis, and peripheral neuritis, which parallel with lesions seen in Lyme disease patients.1,3,4 There are several factors that should be considered when evaluating phenotypes in this model. For example, disease severity in laboratory mice is influenced by genotype, immune status, age of the mice, Bb strain and passage history of the strain, infectious dose, and route of inoculation.2,4,9 It is well-accepted that inbred mouse strains are equally susceptible to Bb infection, but the susceptibility of arthritis is dependent on the mouse genotype.3
The C3H/HeN and C3H/HeJ mice are often used to study histopathological changes in the joints as these mouse strains develop moderate to severe subacute tenosynovitis in the tibiotarsal joints.3,4 Bb reaches the tibiotarsal joint after 2 weeks of intradermal or subcutaneous inoculation of spirochetes.4 At 4 weeks of infection, the inflammation in synovium, tendon sheaths, and periarticular connective tissue is predominantly composed by neutrophils mixed with moderate numbers of macrophages, fibrinous exudate in joint lumen, and hyperplasia and hypertrophy of the synovium surrounding the tibiotarsal joints.4 BALB/c mice develop mild to moderate tenosynovitis and C57BL/6 mice develop mild tenosynovitis following infection with Bb.4,12 In contrast, SCID mice develop more severe tenosynovitis than immunocompetent mouse strains as SCID mice exhibit higher bacterial loads in the tibiotarsal joints and develop persistent infections in tissues.4 The lesions seen in the tibiotarsal joints of SCID mice infected with Bb are characterized by marked hyperplasia and hypertrophy of synoviocytes with multifocal moderate to marked neutrophilic to histiocytic inflammation in synovium, ligaments, and tendon sheaths (e.g., tibiotarsal extensor tendon), fibrin and proteinaceous exudate.3,4 As shown in this case the lesions are commonly noted at 4 weeks of infection but can persist for 8 weeks post-inoculation when mice SCID mice are inoculated with high doses of Bb. Spirochetes are readily visible by silver stains and/or immunohistochemistry, which are often observed within the proliferating synovium and associated with neutrophils and macrophages. The distal tibia in SCID mice often exhibits areas of periosteal remodeling and endochondral ossification with new bone formation.3,4 Occasionally, severe lesions in SCID mice can develop progressive pannus in joint spaces with regional inflammation and destruction of articular cartilage and subchondral bone.4
The use of mouse strains deficient in component of the acquired immune system have been instrumental to understand their role in host defense against Bb infection. Several studies have demonstrated the importance of B cell activation and immunoglobulin production in the control of Bb infection in vivo. (6, 13, 20) Mice deficient antibody production, such as µMT-/- mice (lack B cells but bear T cells) and SCID and rag1-/- mice (lack B and T cells) developed persistent tenosynovitis and carditis and exhibited an elevated bacterial burden in tissues. (6, 13, 20) In contrast, studies with mice lacking all T cells (TCR β/δ -/-) or only αβ TCR-expressing cells, or with mice depleted of CD4 or CD8 T cells, have shown a minor role for T cell-deficiency during course of Bb infection. (20)
The signaling pathway involved for the enhanced severity of tenosynovitis in C3H mice was identified by global gene expression analysis in Bb-infected tibiotarsal joints. Miller et al., demonstrated that type I interferons were upregulated in the tibiotarsal joints of C3H mice at 1 week of Bb infection, 2-3 weeks prior the development of subacute tenosynovitis in this mouse.14,15 The peak of type I interferons (IFN-α/β) likely occurred prior to infiltration of lymphocytes into the joints lesions of C3H mice.14,20 The involvement of type I interferons in tenosynovitis was subsequently confirmed by either the systemic administration of a type I interferon receptor (IFNAR-1) blocking antibody or the ablation of the IFNAR1 in mice, which resulted in suppression of type 1 interferons and tenosynovitis in tibiotarsal joints of mice.14,20 The early upregulation of IFN-responsive transcripts was absent in the joints from tenosynovitis resistant C57BL/6 mouse.14 Instead, Bb infected C57BL/6 mice displayed an increased expression in genes involved in epidermal differentiation, cell adhesion, cell-cell interaction, and wound repair.14,15 Additionally, IL10-/- mice on the C57BL/6 background showed several IFN-inducible transcripts were markedly upregulated in the joints at 2 weeks of infection and exhibited increased tenosynovitis severity.18 Infected IL-10−/− mice also exhibited upregulation of inflammatory mediators, such as IFN-γ, CXCL9, and CXCL10 and tibiotarsal joints were infiltrated with NK cells, NKT cells, CD4+ T cells, and macrophages.18
Unlike most classical gram-negative bacterial pathogens, Bb does not produce LPS or toxins and lacks a specialized secretory system. Instead, the Bb genome encodes numerous surface lipoproteins that allow spirochetes to adapt, adhere, invade, and/or persist in tissues.17 These surface proteins are recognized by TLR2 receptor and induce a proinflammatory response via MyD88 signaling pathway.8,17 In addition, CD14 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein expressed on macrophages/monocytes that serves as a co-receptor for TLR2 to facilitate the activation of the innate immune response against Bb.8 Previous studies have shown that the loss of either TLR2 or CD14 in mice leads to a defective innate immune response against Bb, and both TLR2-/- and CD14-/- mice showed an increased severity of tenosynovitis.5,21 There are several inflammatory mediators, such as prostaglandins, leukotrienes, neutrophil recruiting chemokine (KC) among others, in which the use of targeted gene-knockout mouse strains have demonstrated their role in modulating tenosynovitis in Bb-infected mice.6,16 In addition, classical forward genetic experiments using intercross populations between C3H and B6 mice led to the identification of multiple Bb arthritis–associated (Bbaa) quantitative trait loci (QTL) on five different chromosomes of the mouse (chromosome 1, 4, 5, 11 and 12).19
Natural bacterial infections resulting in primary arthritis in laboratory mice are rare, but arthritis can occur as result of septicemia and/or local opportunistic bacterial infections in the joints. Septic arthritis has been described in laboratory mice infected with Streptobacillus moniliformis and Corynebacterium kutscheri.10 Monoarticular pyogenic arthritis may occur as result of opportunistic bacterial infections due to cutaneous abrasions and lacerations infected with opportunistic bacteria.10 Bacterial agents that can cause suppurative arthritis in laboratory mice include Staphylococcus aureus, Group B Streptococcus spp. Rodentibacter pneumotropica, E. coli, and Klebsiella pneumoniae spp. Experimental infections with Mycoplasma pulmonis can cause polyarthritis in experimentally inoculated B cell-deficient mice and SCID mice, and immunocompetent C3H/HeN mice.10 However, arthritis is not a significant feature in natural M. pulmonis infections in mice.10 M. pulmonis induced arthritis is characterized initially by suppurative inflammation affecting primarily the carpal and tarsal joints and associated tendon sheaths. As the process becomes chronic, lymphocytic cell infiltration and synovial hyperplasia are common histological features in the affected joints.10
Contributing Institution:
Laboratory of Comparative Pathology, Memorial Sloan Kettering Cancer Center, The Rockefeller University, Weill Cornell Medicine.
https://www.mskcc.org/research/ski/core-facilities/comparative-medicine-pathology-0
JPC Diagnosis:
Tarsal joint: Tenosynovitis, proliferative and neutrophilic, chronic, diffuse, severe with focal bony lysis and periosteal new bone growth
JPC Comment:
We were excited to finally utilize this case as it has been waiting on our shelf for several years for a moderator willing to jump into the details that are so painstakingly crafted. Experimental disease cases can be difficult to read cold as JPC conference participants only get the tissue and species in advance of the conference date, and a single HE section without access to the special stains that demonstrate the presence and morphology of an infectious agent.
Many conference participants considered neoplasms such as histiocytic sarcoma and osteosarcoma as primary differentials in this case given the involvement of the cortical bone and the extensor tendon. That said, the absence of mitotic figures and matrix and the presence of many neutrophils are more suggestive of granulation tissue than neoplasia. Periosteal new bone formation reflects periosteal irritation and could support either bony infection or neoplasia. Some participants considered the potential for an induced neoplasm (i.e. osteosarcoma) secondary to inflammation, though the role of Bb in neoplasia appears to be limited to a short description in certain human breast cancers.22
Conference participants also discussed “pannus” in the context of this lesion. “Pannus” describes the spread of fibrovascular or granulation tissue across multiple organs including the cornea, joint surfaces, and the endothelium. In a veterinary context, this can be confusing as chronic superficial keratitis (also referred to as “pannus”) is more commonly seen in primary practice than erosive polyarthritis. Additionally, veterinary literature seldom uses the term pannus to describe thickening of the joint and/or bony/cartilaginous involvement in osteoarthritis cases. Murine tissue transplantation, particularly with SCID mice, may be one exception to this observation.23
References:
- Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162: 133-138.
- Barthold SW. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991;163: 419-20.
- Barthold SW, Sidman CL, Smith AL. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47: 605-613.
- Barthold SW, Cadavid, D., Philipp, M. Animal Models of Borreliosis. S Samuels and J Radolph, In: Borrelia: Molecular Biology, Host Interaction and Pathogenesis, 1st edition, Norfolk, UK: Caister Academic Press; 2010:359-411.
- Benhnia MR, Wroblewski D, Akhtar MN, Patel RA, Lavezzi W, Gangloff SC, Goyert SM, Caimano MJ, Radolf JD, Sellati TJ. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 2005;174:1539-48.
- Bockenstedt LK, Wooten RM, Baumgarth N. Immune Response to Borrelia: Lessons from Lyme Disease Spirochetes. Curr Issues Mol Biol. 2021;42: 145-190.
- Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991; 9:323-50.
- Cervantes JL, Hawley KL, Benjamin SJ, Weinerman B, Luu SM, Salazar JC. Phagosomal TLR signaling upon Borrelia burgdorferi infection. Front Cell Infect Microbiol. 2014; 20; 4:55.
- de Souza MS, Smith AL, Beck DS, Kim LJ, Hansen GM Jr, Barthold SW. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect Immun. 1993;61: 4493-7.
- Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A. Bacterial diseases. In: The Mouse In Biomedical Research. Vol 2, 2nd edition, San Diego, CA USA: El Sevier Inc; 2006: 325-469.
- Franklin CL. Microbial considerations in genetically engineered mouse research. ILAR J. 2006; 47 :141-55.
- Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66: 161-168.
- McKisic MD, Redmond WL, Barthold SW. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J Immunol. 2000;164: 6096-6099.
- Miller JC, Ma Y, Bian J, et al. A critical role for type I IFN in arthritis development following Borrelia burgdorferi infection of mice. J Immunol. 2008;181: 8492-8503.
- Miller JC, Ma Y, Crandall H, Wang X, Weis JJ. Gene expression profiling provides insights into the pathways involved in inflammatory arthritis development: murine model of Lyme disease. Experimental and Molecular Pathology. 2008;85: 20-27
- Pratt CL, Brown CR. The role of eicosanoids in experimental Lyme arthritis. Front Cell Infect Microbiol. 2014; 28:4:69.
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Micro. 2012;10: 87-99.
- Sonderegger FL, Ma Y, Maylor-Hagan H, et al. Localized production of IL-10 suppresses early inflammatory cell infiltration and subsequent development of IFN-gamma-mediated Lyme arthritis. J Immunol. 2012;188: 1381-1393.
- Weis JJ, McCracken BA, Ma Y, et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162: 948-956
- Weis JJ, Bockenstedt, L.K. Host Response. In: Borrelia: Molecular Biology, Host Interaction and Pathogenesis. 1st edition. Norfolk, UK: Caister Academic Press; 2010:413-441.
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002; 168:348-55.
- Gaur G, Sawant JY, Chavan AS, et al. Effect of Invasion of Borrelia burgdorferi in Normal and Neoplastic Mammary Epithelial Cells. Antibiotics (Basel). 2021 Oct 24;10(11):1295.
- Liu S. Human Xenograft Model. Methods Mol Biol. 2024;2766:9-15.