Signalment:
Signalment:
5-year-old, female, Spanish-Boer cross
goat (Capra hircus)This animal was noted to be acutely disoriented, visually
impaired, and intermittently down and twitching. It responded immediately to
empirical tr-eatment, but never completely recovered full neurological function
and was euthanized two months later.
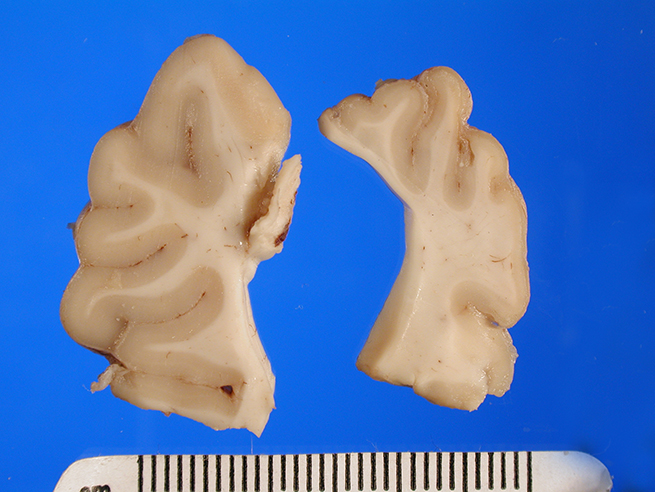
Gross Description:
The brain appeared somewhat shrunken on
removal from the cranial vault. The cerebral cortex was thinned and discolored,
especially in the occipital-parietal region, with areas of clefting and separation
from the underlying white matter noted.
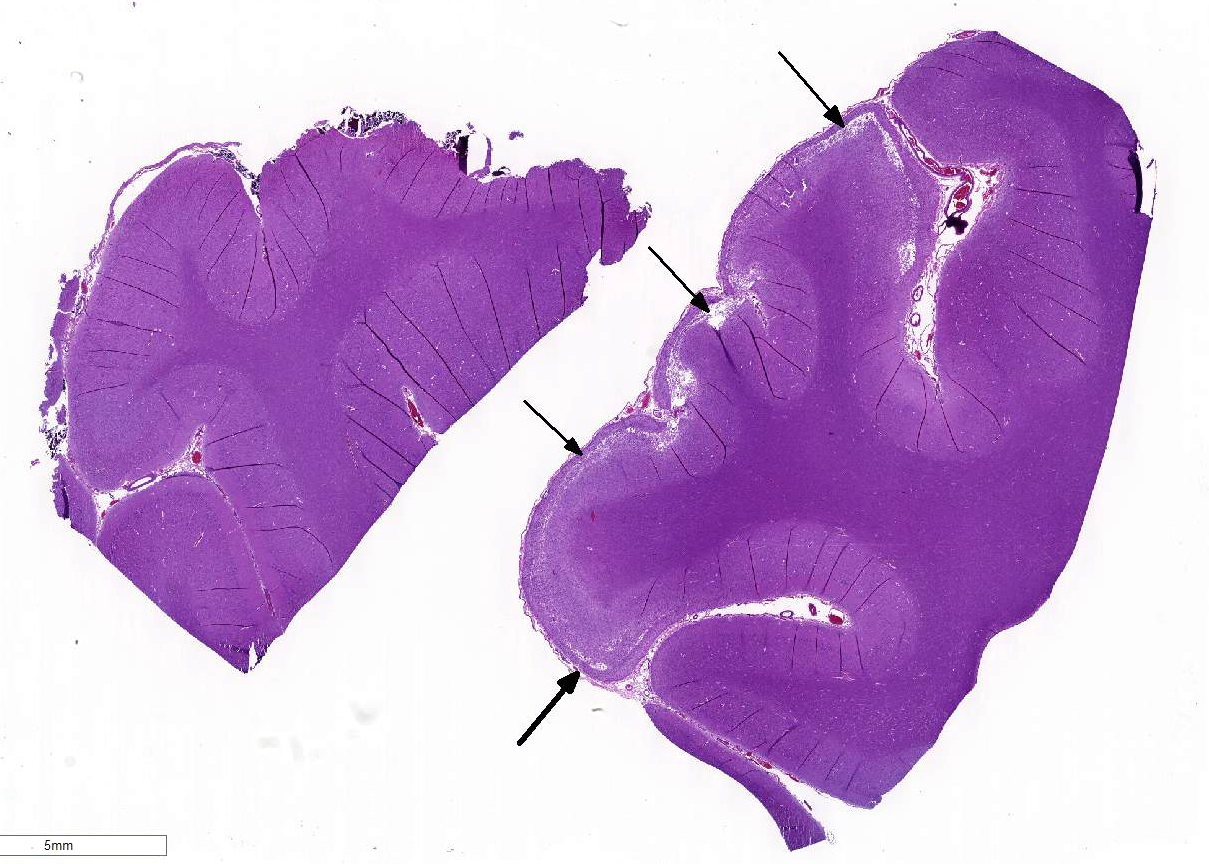
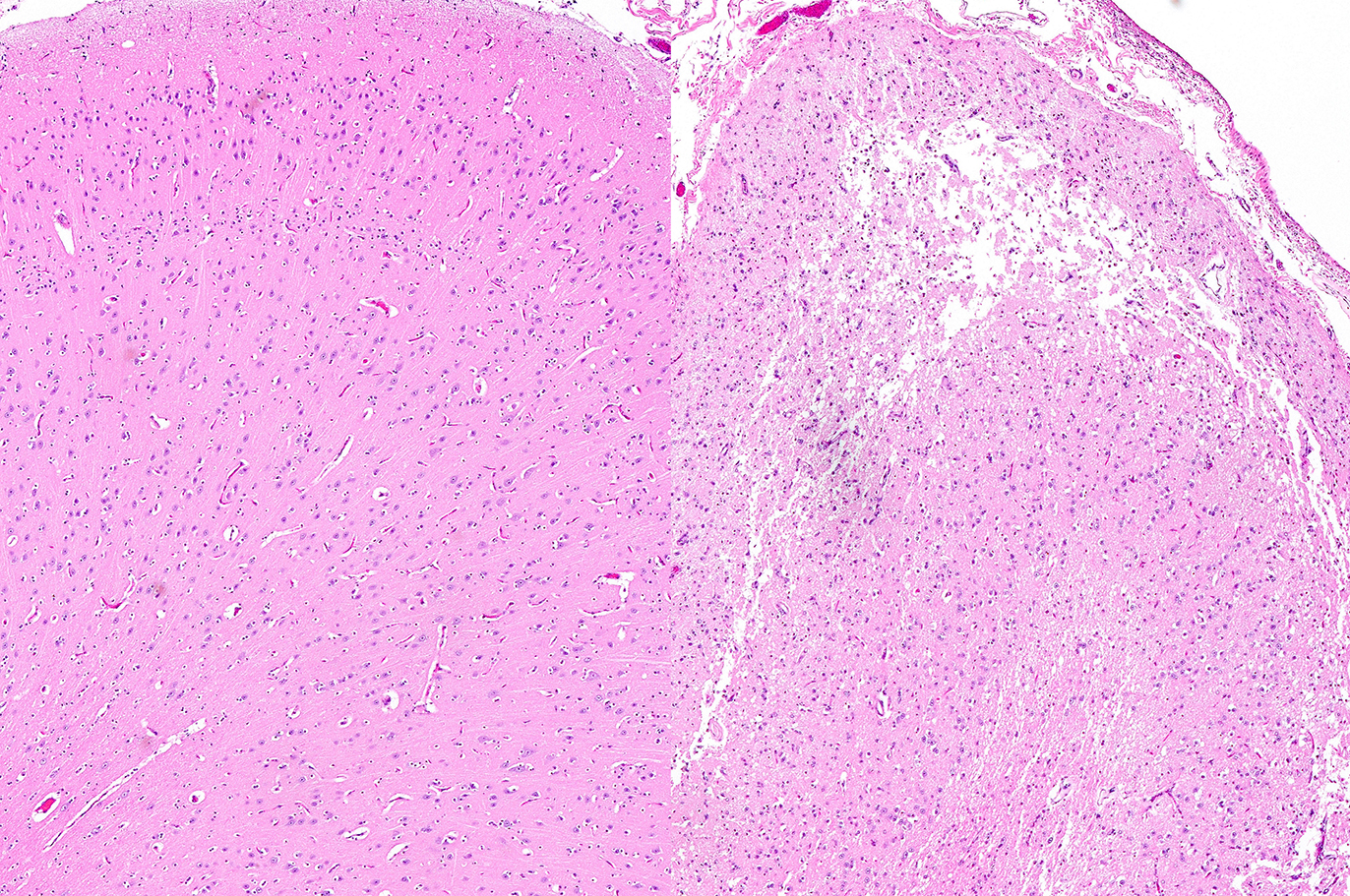
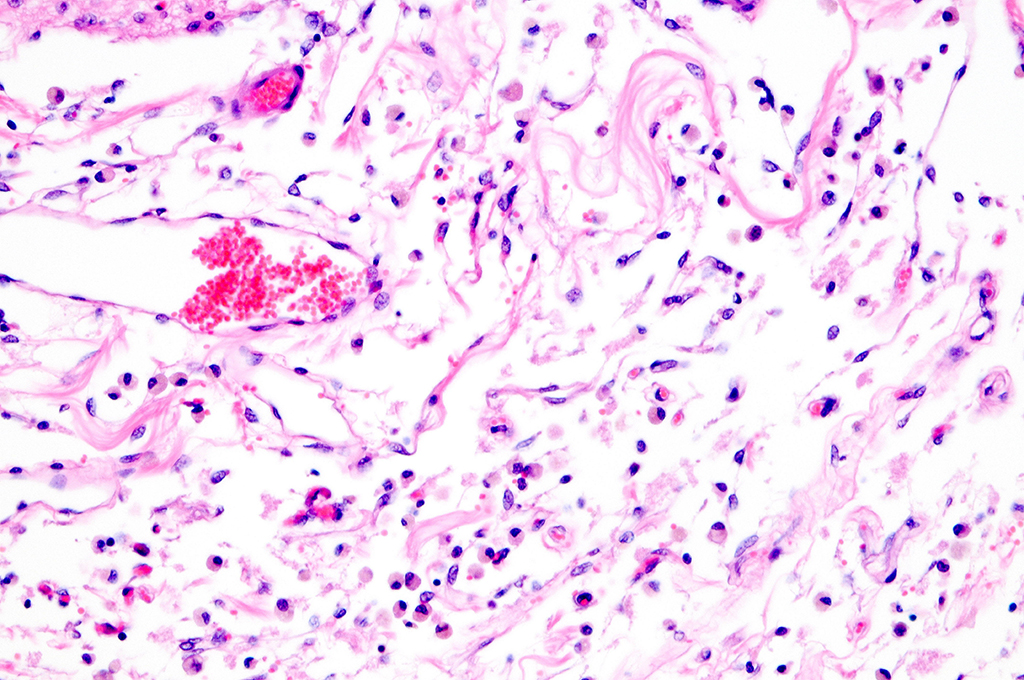
Histopathologic Description:
Sections of occipital-parietal cortex are examined. Each slide
contains tissue from the affected animal as well as location-matched cortex
from an age-matched control goat. The latter is essentially normal brain for
comparison purposes. In the former, there is focally extensive laminar loss of
cortical grey matter, significantly di-minishing cortical thickness. Although
occasional neuronal cells remain present, residual cellularity consists
primarily of large, reactive (gemistocytic) astrocytes, activated microglial
cells, and phag-ocytically active macrophages (Gitter cells). Capillary
structures are prominent with somewhat swollen endothelium, both in grey matter
and in collapsed, redundant leptomeninges present in the expanded subarachnoid space.
Abundant, phagocytically active macrophages are also noted in the latter. The white
matter appears slightly hypercellular, probably due to a mild reactive
astrocytosis also present in this region. Infrequent pyknotic cells, probably
representing necrotic oligodendroglia, are seen due to axonal die back. Mild
perivascular lymphocytic cuffing is noted in white matter in some sections.
Morphologic Diagnosis:
1) Subtotal laminar cortical necrosis and collapse, severe, with
marked extensive residual reactive gliosis, vacuolization and patchy regions of
parenchymal se-paration/clefting, with areas of tissue dropout and meningeal
collapse
2) Patchy, mild perivascular lymphocytic cuffing, subjacent white
matter, mild (some sections)
Lab Results:
None.
Condition:
Polioencephalomalacia (PEM)
Contributor Comment:
The
microscopic findings are consistent with po-lioencephalomalacia (PEM). This is
a morphological term used to describe necrosis with softening (malacia) in grey
matter of the brain. The condition is described in a surprisingly wide range of
domestic animals, both ruminant and carnivores, as well as some non-domestic
species.
9 Wernicke`s encephalopathy is the equivalent human disease,
which is classically associated with chronic alcoholism. Cattle, sheep and
goats are commonly affected ruminants, although a variety of other species are
also susceptible. Clinical manifestations of the disease are variable, with
animals often presenting with facial twitching, teeth grinding, sal-ivation,
blindness, seizures and opisthotonus.
8 The condition affects primarily
young animals, and sheep and goats, as a rule, have a shorter course with fewer
survivors. The syndrome is not always fatal; mortality rates are reported at
50-90%, although surviving animals typically have significant neurological
deficits, including visual impairment and stupor.
4 Disease is seen
worldwide and is responsible for important economic losses in many countries.
The condition is more commonly seen in goats under intensive management
conditions when fed more grain concentrate to encourage accelerated growth.
7
PEM
was recognized as a clinical and pathological entity long before specific
pathogeneses had been discovered. Originally applied as a diagnosis to cattle
and sheep losses in Colorado, the mor-phological designation of cerebrocortical
malacia was subsequently used sy-nonymously for the specific entity of thiamine
deficiency disease. However; it is now known that many cases of PEM in
ruminants cannot be ascribed to thiamine deficiency.
2,3,6 There is
often a lack of changes in thiamine concentration in ruminal fluid, tissue and
blood in affected animals. Furthermore, there has been a failure to induce the
disease by ex-perimentally created deficiencies. The most compelling argument
for thiamine`s role in PEM had been that administration of it in clinical
cases, especially to those early in the disease course, often resulted in
recovery. However, this is now believed to be related to improved energy
metabolism in the impaired brain, regardless of the inciting cause.
5, 6, 7
Currently, it is
believed that PEM in ruminants can involve a wide range of pathogeneses,
including toxic, metabolic, dietary/nutritional and even infectious events. In
addition to thiamine deficiency, some of the specific causes of
polioencephalomalacia in ruminants include sulfur poisoning, lead poisoning,
salt poisoning (water deprivation), ad-ministration of levamisol or thiamine
analogues such as amprolium, ingestion of thiaminase rich plants, and infection
with bovine herpesvirus.2
Gross pathological
changes are often striking, with the parietal-occipital cortex being most
prominently affected. In acute cases, brains may have a swollen appearance and
palpable softness, with flattening of gyri and narrowing of sulci. With more
prolonged survival, as in this case, there is marked thinning or, in some
areas, complete absence of friable, necrotic appearing grey matter, with zones
of clefting/separation from underlying white matter visible.8 The
subarachnoid space is widened, and brains often appear smaller or shrunken.
Areas of cerebrocortical necrosis (CCN) can be identified by autoflourescence
under UV light, as a consequence of degraded lipoidal material within
macrophages or high molecular weight collagen-like material. Although blood
pyruvate levels may be elevated,4 other serum biochemical analysis
is variable and is generally of little value in disease diagnosis.
As
previously noted, the animal in question responded favorably to thiamine
administration upon onset of signs, but continued to have significant
neurological and visual deficits after stabilization.
JPC Diagnosis:
Brain, cerebral
cortex:vNecrosis, laminar, multifocal to coalescing, with reactive gliosis.
Conference Comment:
The contributor
provides an excellent review of po-lioencephalomalacia, and the aged-matched
control provided on the slide increases the teaching / learning value of this
case. In ruminants, polioencephalomalacia is usually limited to the
cerebrocortical grey matter and has a laminar pattern of distribution, often
being referred to as "laminar cortical necrosis." The lesion in ruminants
discussed above shares similarities with salt poisoning in swine and has been
documented in cases of lead poisoning in cattle as mentioned in WSC Case 3 of
this conference. It is most often a disease of young animals, although older
animals may be affected sporadically.
Lesions of PEM vary in
severity, depending on various factors such as species, age and duration.1
Lesions are more severe grossly obvious in animals that survive for a period of
time. The cerebral cortex often demonstrates superficial, laminar pallor which
will trace the grey-white matter junction and may be most prominent in the
gyri. Lesions are bilaterally symmetrical and are apparently more consistent in
the caudal cerebral hemispheres. The distribution appears to be related to the
area supplied by the middle cerebral artery.1
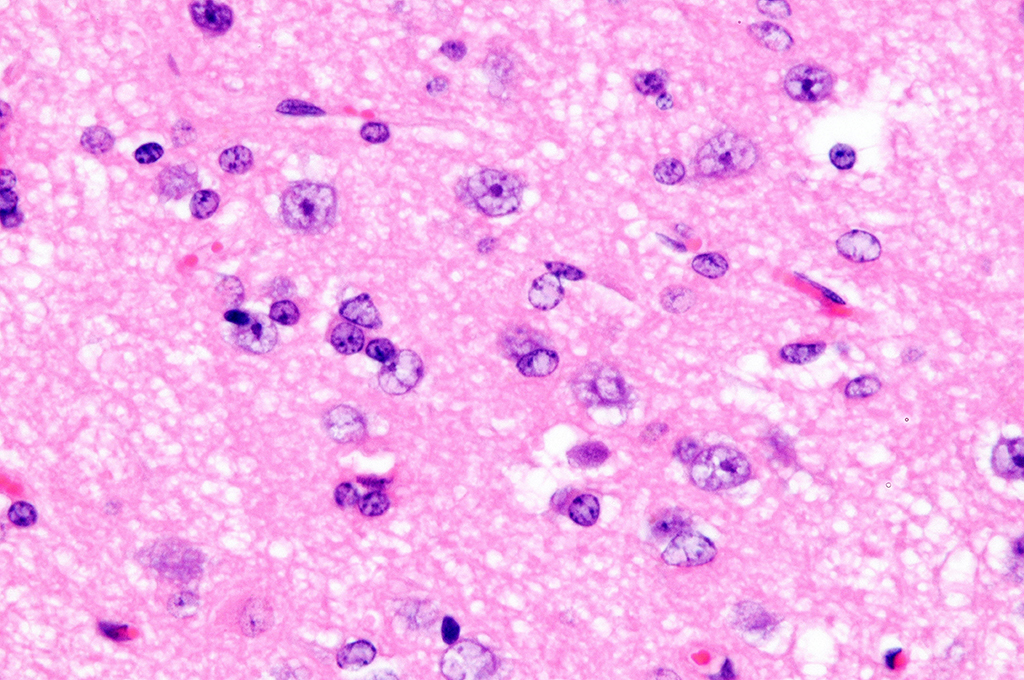
Cerebrum, goat: Adjacent to necrotic areas, small numbers of
glial cells abut neurons. (HE, 400X). (Photo courtesy of: Division of
Laboratory Animal Resources (DLAR) University of Pittsburgh,
http://www.dlar.pitt.edu/
There
is some slide variation in the severity of lesions in this case, but in
general, it is representative of the classic microscopic lesions of PEM. Polioencephalomalacia
does
not have a specific etiology, as discussed above, but is often directly or
indirectly linked to a deficiency in thiamine. Sulfur-containing compounds
have also been implicated in some cases of PEM.1 There is some
question regarding the observed tissue autoflourescence in cases of PEM with
some references stating it may originate from substances in mitochondria as
opposed to ceroid-lipofuscin
pigments.10
References:
1. Cantile C, Youssef S. Nervous
system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of
Domestic Animals. 6th ed. Vol 1. St. Louis, MO: Elsevier; 2016:309-312.
2. Fabiano
JF de Sant'Ana, Claudio SL Barros. Polioencephalomalacia in ruminants in
Brazil. Braz J Vet Pathol. 2010; 3(1):70-79.
3. Gould
DH, Polioencephalomalacia. J. Animal Science. 1998;76: 309-314.
4. Koestner
A, Jones TC. The Nervous System. In: Jones TC, Hunt RD, King NW ed. Veterinary
Pathology. 6th ed. Philadelphia: Williams & Wilkins; 1997: 1272-1274.
5. Najarnexhad
V, Aslani MR, Balali-Mood Mehdi. The therapeutic potential of thiamine for
treatment of experimentally induced subacute lead poisoning in sheep. Comp
Clin Patho. 2010; 19:69-73.
6. Niles
GA, Morgan SE, Edwards WC, The relationship between sulfur, thiamine and
polioencephalomalacia – a review. Bovine Practice. 2002; 36: 93-99.
7. Smith
MC, Sherman DM ed. Goat Medicine. 2nd ed. Ames, IA:Wiley Blackwell;
2009: 222-226 .
8. Sullivan
ND. The Nervous System. In: Jubb KVF, Kennedy PC, Palmer N eds. Pathology of
Domestic Animals 3rd ed. Vol 1. New York: Academic Press, Inc.; 1985:
251-256.
9. Summers
BA, Cummings JF, de Lahunta A. Veterinary Neuropathology. New York:
Mosby; 1995:277-280.
10. Zachary JF. Nervous System. In: McGavin MD, Zachary JF, eds. Pathologic
Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier;
2012:851.