Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 2
September 5th, 2018
CASE I: 21-168-14/12-168-17 (JPC 4019882).
Signalment: 18-month-old male ferret (Mustela putorius furo)
History: This ferret was first initially diagnosed with ferret granulomatous coronavirus-associated disease at about six months of age at the private veterinary referral clinic. Initial clinical findings at that time included diarrhea, lethargy, anorexia, and a 4 cm mesenteric mass. Biopsy of this mass confirmed a diagnosis of granulomatous coronavirus-associated disease. The ferret was maintained by supportive care including occasional antimicrobial therapy to treat diarrhea and lethargy, sucralfate, potassium supplements, and hand feedings of highly palatable soft foods. After continued decline over many months, the ferret was euthanized one year after the initial diagnosis and the carcass submitted for gross and histopathological examination.
Gross Pathology: Gross postmortem findings included multiple firm tan 1-5 m diameter nodules throughout the mesentery, bilateral pale cortical pitting lesions on the kidneys, multifocal coalescing raised white nodules on the lungs with complete consolidation of the accessory lobe, and splenomegaly with pinpoint pitted lesions.
Laboratory results: CBC and serum chemistry analysis performed at a commercial diagnostic laboratory revealed anemia (PCV 23%, reference, 43-55), leukocytosis (14x103/um/L) with 89% lymphocytes, mild hypoalbuminemia (1.6 g/dL; reference, 2.6 to 3.8 g/dL) and mild hyperglobulinemia (3.6 g/dL; reference, 1.8 to 3.1 g/dL). Abdominal radiographs revealed a poorly defined area of increased opacity in the midabdomen apparent on the lateral but not ventrodorsal views.2
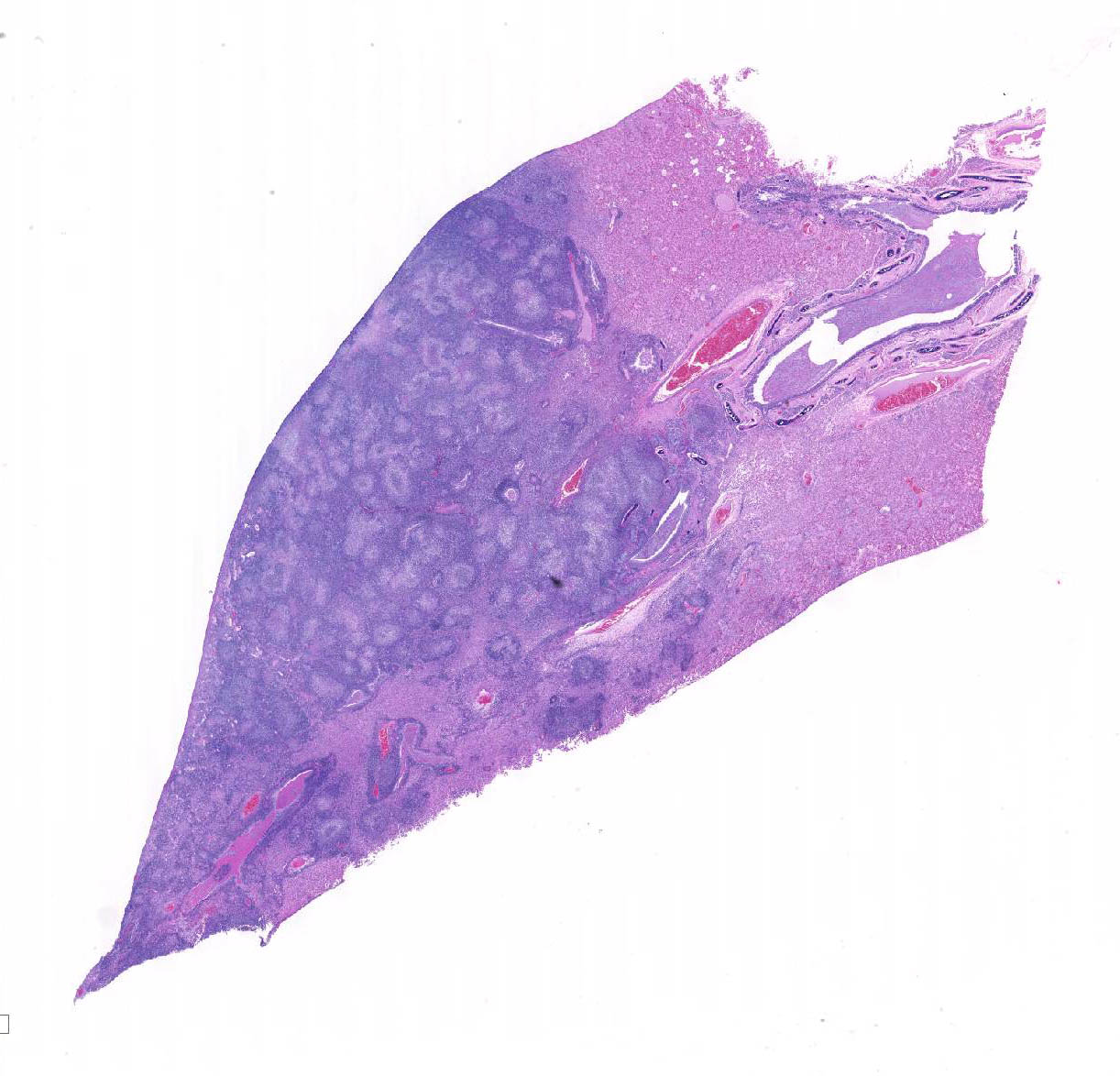
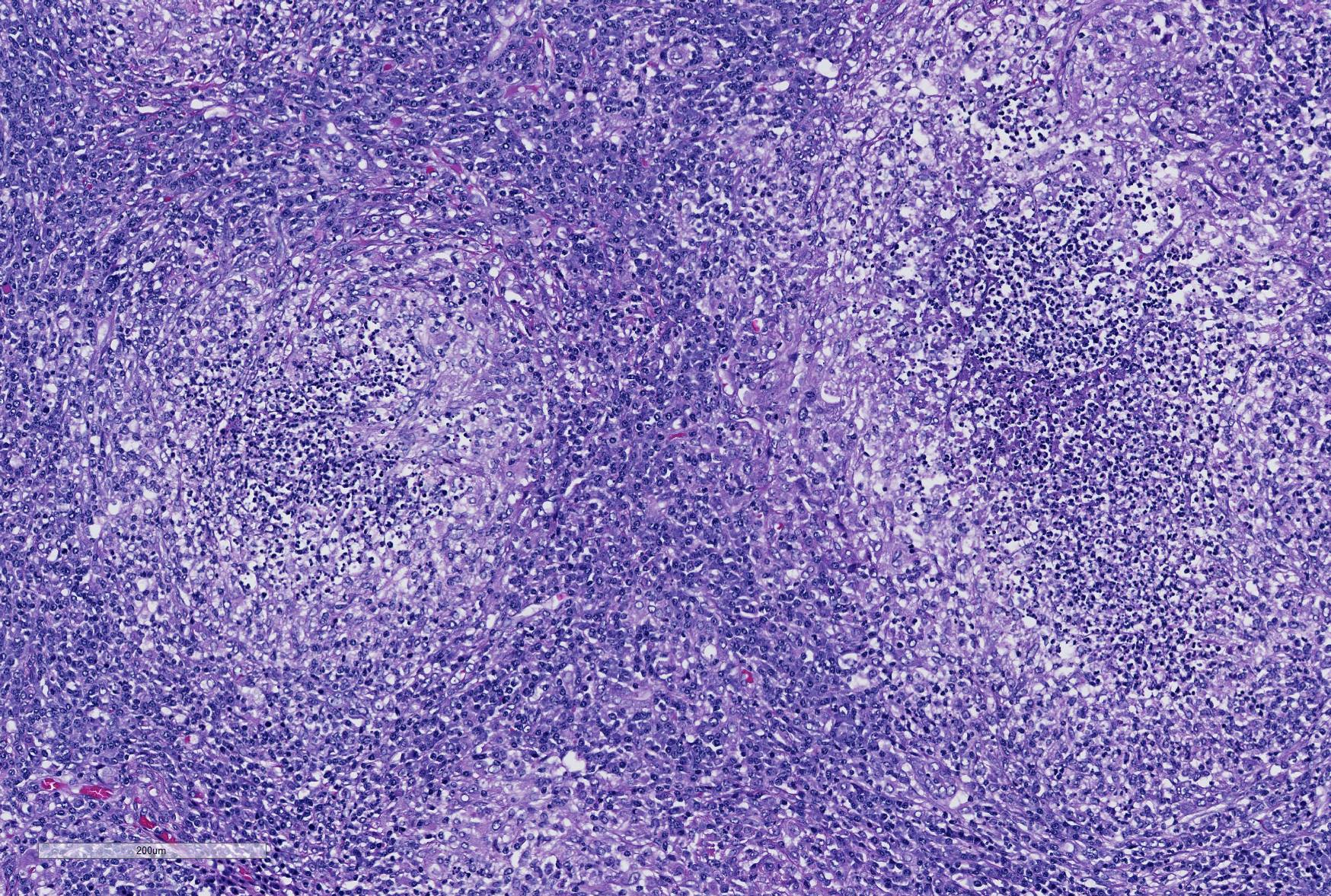
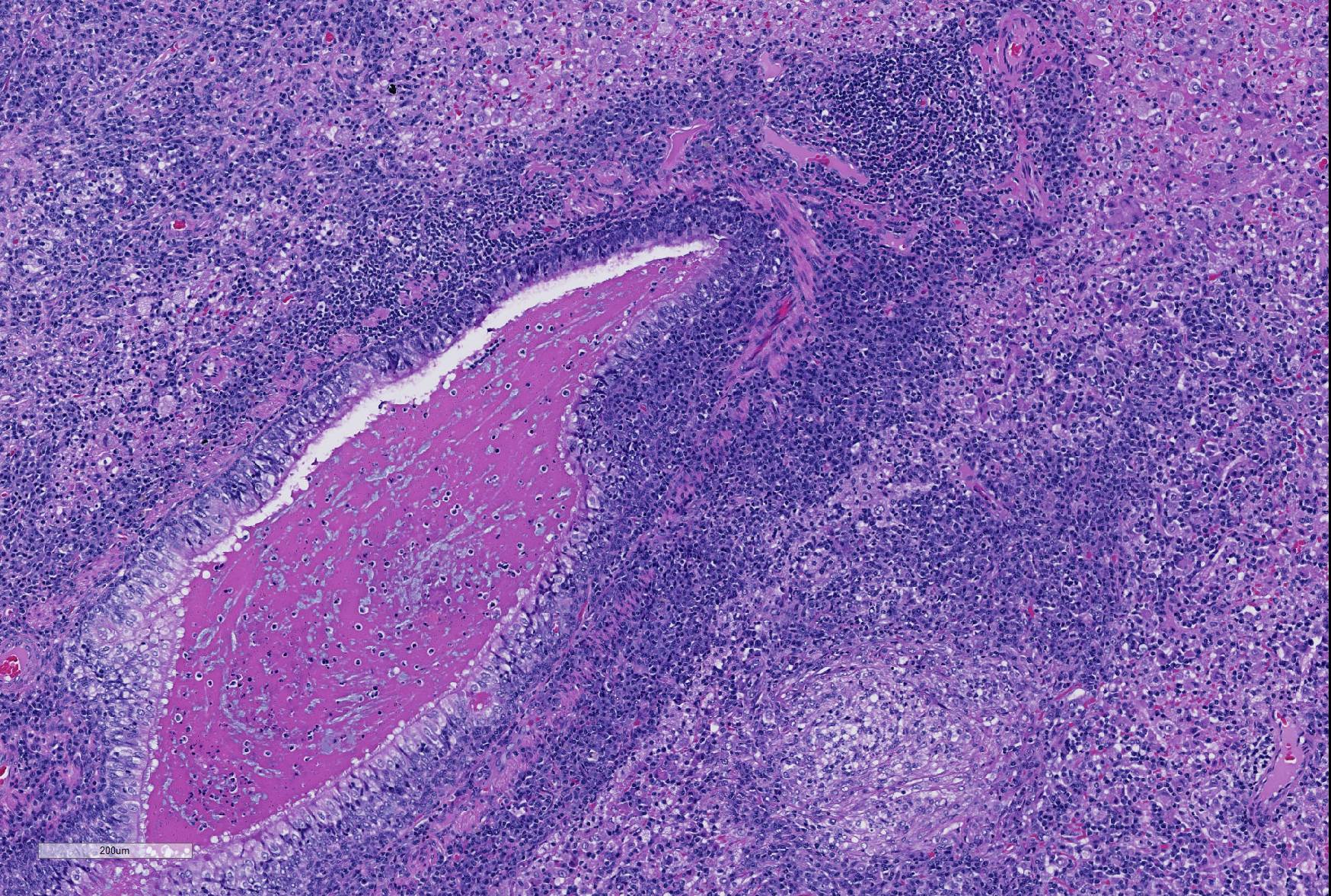
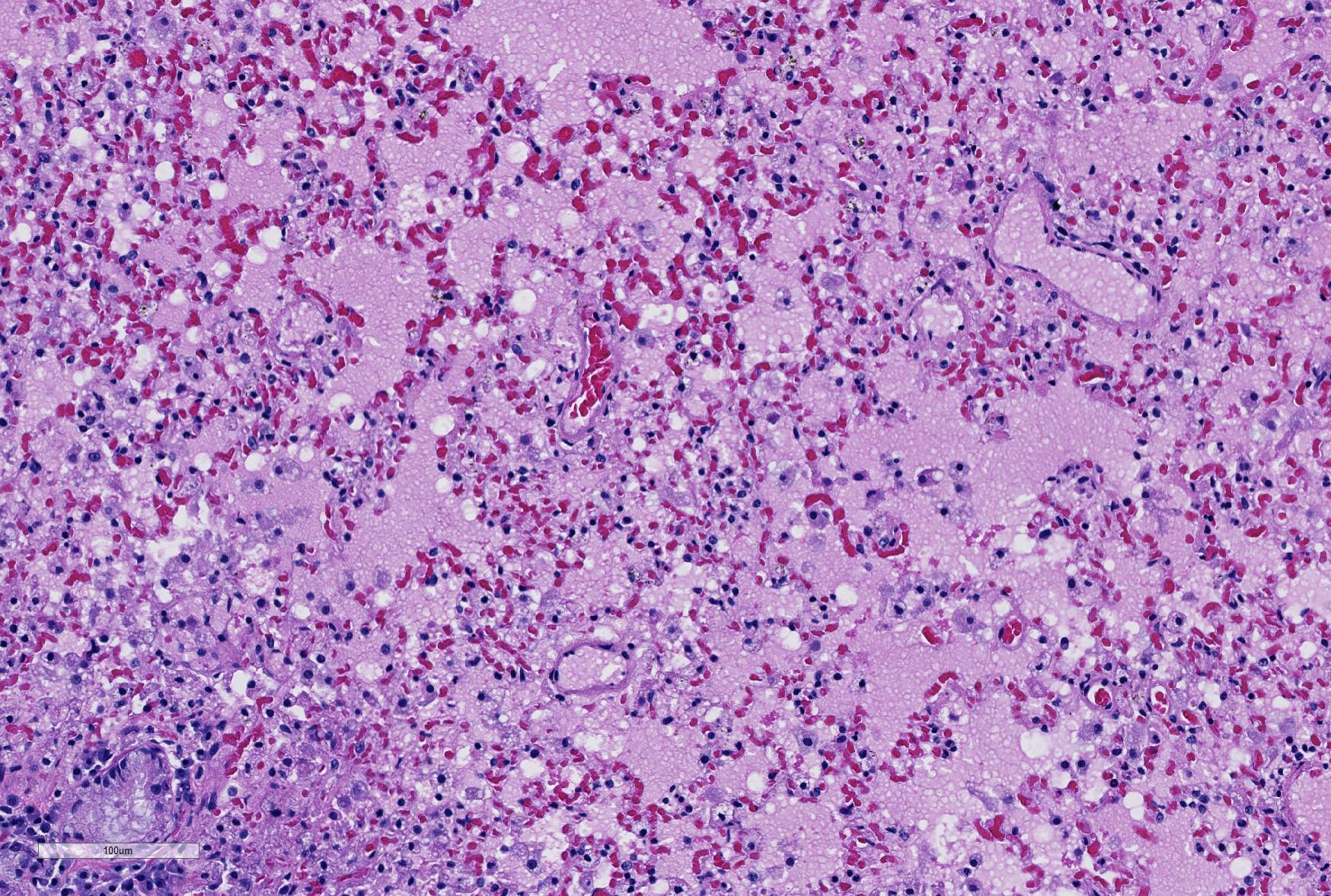
Microscopic Description: Lung: Depending upon the section examined, there is either marked diffuse or severe large segmental effacement and expansion of the normal pulmonary parenchymal architecture by variably sized discrete and fairly well organized nodular foci (granulomas). The granulomas/granulomatous foci are comprised of a central variable sized core of necrotic cellular debris surrounded by numerous large epithelioid macrophages with indistinct cell borders, abundant granular to vacuolated to clear cytoplasm, and large oval indented to marginated nucleus. The macrophages are in turn encircled by numerous plasma cells, lymphocytes and fewer neutrophils, which are in turn encircled by fibroblasts and variably thick band dissecting fibrous connective tissue. Variably within the sections, sparse to abundant non–staining clear needle like acicular crystals (cholesterol clefts) are often associated closely with multinucleated (macrophages) giant cells (Langhans’ and foreign body type) within the granulomatous foci. The remaining discernible alveolar septa and lumen are expanded by numerous type II pneumocytes and large epithelioid macrophages, variable numbers of plasma cells/lymphocytes, degenerate neutrophils and necrotic cellular debris and low to abundant intraluminal proteinaceous material (edema). The bronchial and bronchiolar lumen when discernible are filled with eosinophilic proteinaceous material, mucous, epithelial and inflammatory cellular debris, associated variable bronchial epithelial hyperplasia, dense peribronchial aggregates of lymphocytes and plasma cells. In many regions, the bronchioles are obliterated by fibrosis or are severely necrotic and replaced by inflammatory cellular debris. There is also prominent lymphoplasmacytic and histiocytic perivasculitis (mostly perivenular), and in some instances, vasculitis characterized by adventitial and medial expansion by edema and inflammatory cells and/ or mild mural/degeneration/necrosis. Multifocal moderate alveolar septal necrosis and interstitial and pleural fibrosis is also noticed. Rarely, dystrophic calcification and bone formation is noticed within the granulomatous foci.
Special stains:
PAS/ GMS:
- Lung: Negative for fungal organisms
Ziehl-Neelson Acid fast stain
- Lung, mesenteric lymph node, stomach, intestine: Negative for acid fast bacilli.
Electron Microscopy:
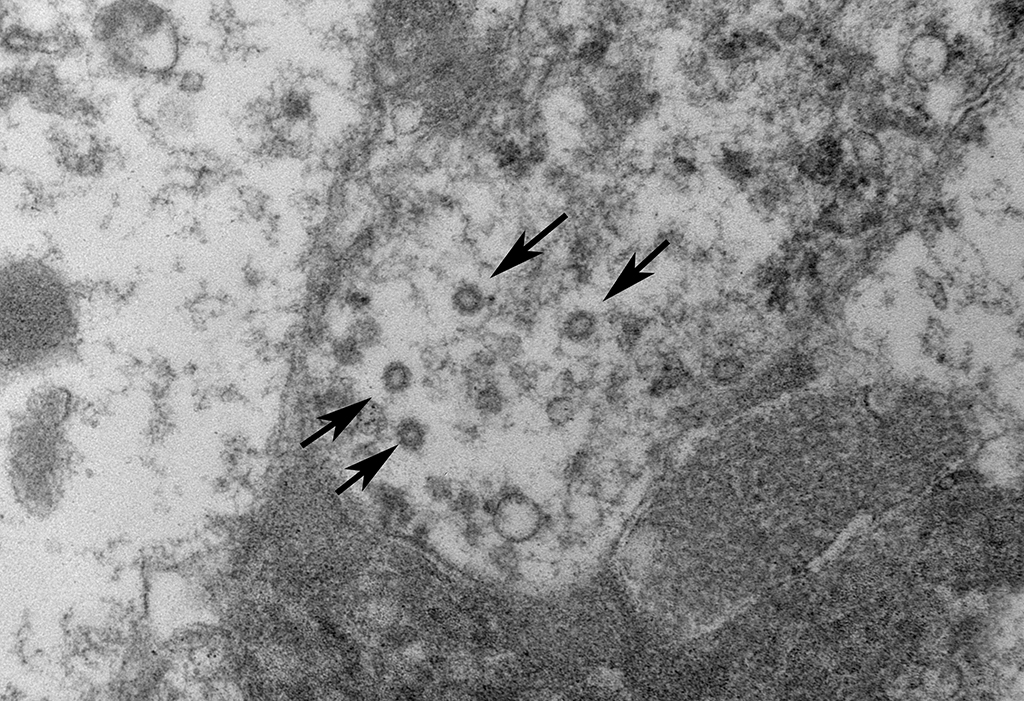
Mesenteric lymph node:
Ultrastructurally, coronavirus-like particles were identified in examined tissues inside macrophages, either within cytoplasmic vacuoles or free in the cytosol (Representative images provided).
Contributor’s Morphologic Diagnoses: Lung: Pneumonia, severe, coalescing to diffuse, chronic, organizing, necrogranulomatous with multinucleated giant cells, cholesterol clefts, pulmonary edema and fibrosis.
Contributor’s Comment: This case is an interesting example of the recently recognized ferret systemic coronavirus-associated disease resembling feline infectious peritonitis (FIP)-like disease syndrome in ferrets. This relatively new disease entity in ferrets was first recognized in Europe in late 2005-early 2006 5,8 and since then has been systemically characterized in the US 5,12,17 and more recently identified in Japan9. The clinical disease and the lesions in all these described cases are similar to those observed with the “dry” form of FIP.1 The differentials considered for this case include mycobacteriosis and systemic fungal infections, however no acid–fast bacilli nor fungal organisms were identified.
Coronaviruses are enveloped, pleomorphic, positive-strand RNA viruses with a diameter or 60-220 nm and classified under the genus Coronavirus within the family Coronaviridae, order Nidovirales.1 The viral particles typically develop in the cytoplasm of affected epithelial cells and macrophages inside vacuoles and form characteristic radiating peplomer spikes on the surface of the envelope, a diagnostic feature in EM,5,8-9,16
Ferret coronaviruses can cause two distinct clinical conditions, namely epizootic catarrhal enteritis (ECE) in association with ferret enteric coronavirus (FECV)8,12,15-16 and the more recent ferret systemic coronal viral disease caused by ferret systemic corona virus (FRSCV) with a clinical picture resembling the “dry” form of feline Infectious peritonitis (FIP)5,12,16. ECE was first observed in the spring of 1993 on the East Coast of United States and a detailed description of the disease and its association with corona virus was first published in 2000.15 Unlike FRSCV associated FIP-like disease in ferrets, ECE affects all age groups and is more severe in aged ferrets.15 ECE is primarily an enteric disease with lesions consistent with intestinal coronaviral infection such as vacuolar degeneration and necrosis of villous enterocytes, villous atrophy, blunting and fusion, and lymphocytic enteritis.8,12,15-16
Feline coronavirus (FCoV) typically infects domestic and exotic felids. Two biotypes of FCoV include feline enteric coronavirus (FECV) and feline Infectious peritonitis virus (FIPV). FECV replicates in enterocytes causing clinical disease resulting in diarrhea or an asymptomatic infection, whereas FIPV replicates in macrophages resulting in a systemic infection. Both “wet” and “dry” forms of feline infectious peritonitis (FIP) occur depending upon the immune status of the animal. Histologically, pyogranulomatous inflammation involves multiple tissues predominantly with a vasculocentric distribution targeting small arterioles and venules are hallmarks of this disease.1 In a similar fashion, ferret systemic coronaviral disease also causes pyogranulomatous to granulomatous lesions and /or vasculitis in multiple organs including the spleen, mesenteric lymph nodes, intestine, kidneys, brain etc. The common clinical signs associated with the systemic form include anorexia, weight loss, diarrhea and large palpable intraabdominal masses. Less frequent findings included hind limb paresis and central nervous system signs. Immunoreactivity with FIP feline coronaviral antigen (FCoV) on paraffin sections and/or serum samples has been demonstrated by immunohistochemistry in all these cases within the granulomas.5,9,12,15 It is thought that the positive immunoreactivity is due to cross-reactivity from common antigenic determinants within different host specific corona viruses.
In this case, in addition to the lung lesions, discrete granulomas or coalescing granulomatous inflammatory foci were also observed in the mesenteric nodes, mesentery, pancreas, intestinal serosa and the spleen. Cholesterol clefts were not observed within these tissues and multinucleated cells were also sparse. It is of interest to note that cholesterol clefts and the associated presence of numerous multinucleated cells are not typical of earlier descriptions of this disease and even in these cases it was primarily a feature of the lung lesions only and the underlying pathophysiological basis is unclear. Immunohistochemistry for feline coronaviral antigen (FCoV) was not performed on this case for confirmation time of submission. However, on electron microscopic evaluation of the mesenteric lymph node granulomas, enveloped coronavirus-like particles, some with radiating spikes were identified within macrophages, either inside cytoplasmic vacuoles or free in the cytosol.
In this individual, the kidneys exhibited predominant plasmacytic interstitial nephritis with occasional granulomatous foci and severe chronic glomerulonephritis and mild perivasculitis. The distribution and nature of the lesions can mimic those seen with chronic Aleutian disease (AD) caused by distinct parvoviruses in minks and ferrets. AD is characterized by progressive wasting, weight loss, hypergammaglobulinemia, plasmacytosis, interstitial pneumonia in young kits, chronic glomerulonephritis, lymphoplasmacytic interstitial nephritis and prominent arteritis involving kidneys and other organs.13-14 However, in this case we did not have information on the tests for immunoglobulin levels nor additional serology or viral diagnostic tests to rule out a concomitant chronic Aleutian disease.
JPC Diagnosis:
- Lung: Pneumonia, interstitial, pyogranulomatous, multifocal to coalescing, severe.
- Lung: Bronchopneumonia, neutrophilic and necrotizing, multifocal, mild, with marked BALT hyperplasia and intrabronchiolar proteinaceous material.
- Lung: Pneumonia, interstitial, histiocytic, multifocal, mild, with intraalveolar Pneumocystis trophozoites and cysts.
- Lung, subpleural alveoli: Histiocytosis, focally extensive, mild (endogenous lipid pneumonia.)
Conference Comment:
Since the submission of this case and 2012 for consideration in the Wednesday Slide Conference, there has been extensive additional work in the investigation of this important disease of both patent and laboratory ferrets. This particular case itself was published as part of a 5 case study by Autieri et al. in 2015.2 This review was composed of animals from multiple sources including private pets as well as laboratory animals from accredited facility. In addition to identifying cases from seven countries on four continents. The literature review for this publication also identified an interesting report from Denmark in 195111 of a syndrome in 142 ferrets, many less than a year of age, with strikingly similar clinical signs and pathologic findings. The syndrome, known as ends are wanted malignant granulomatosis, demonstrated granulomatous inflammatory changes in lymph nodes, spleen, and liver and histochemical stains failed to disclose the presence of bacteria, fungi, and protozoa as well as negative cultures for aerobic, anaerobic, and mycobacterial bacilli.11
Continuing investigation of ferret coronavirus-associated disease, which has no definitive treatment and is considered invariably fatal in affected individuals, has resulted in the sequencing of the viral genome of two different FECRV strains in Japan.6 This work has determined that the virus shares 50-69% nucleotide sequencing with known coronaviruses suggest doing that the ferret enteric coronavirus (FRCoV) might be classified as a new species in the genus Alphacoronavirus.6 additionally, and enzyme-linked him enzyme linked immunosorbent assay (ELISA) using recombinant partial nucleocapsid proteins of the FRCoV Yamaguchi-1 strain was developed to establish a method for detection of FRCoV from blood sampling.10 Not surprisingly, as anyone who was worked with this virus can understand, 89% of tested ferrets in Japan have been infected with ferret coronavirus.10
In Spain, Doria-Torr et al3 investigated the inflammatory response and antigen distribution of FRCoV infection and compared it to similar studies of FIP in cats. The group identified four distinct types of granulomas in affected animals including those with necrosis, without necrosis, with neutrophils as a central core, and diffuse granulomatous inflammation, very similar to the inflammatory response demonstrated by cats infected with mutated coronavirus. Close inspection of the slide submitted for this case will demonstrate most, if not all of the various types of granulomas discussed in this paper. The authors3 postulate that the various morphologies might be a consequence of different episodes of viremia, as described in cats with FIP. The authors also noted that vasculitis was an uncommon finding, which has been noted in other reviews of this disease. Other FIP-like syndromes that have recently been described in single case reports since the submission of this case include pyogranulomatous panophthalmitis as well as membranoproliferative glomerulonephritis.
Additional clinicopathologic features of ferret coronavirus infection that bear mention in review includes the progressive nature of this disease of juvenile and young adult ferrets which has an average survival time following diagnosis of 69 days5. The most common clinical signs of affected ferrets include weight loss, a palpable intra-abdominal mass or masses, lethargy and anorexia.5 Hematologic findings are nonspecific for this disease and many may be within normal reference levels; the most common hematologic abnormality in affected animals is a polyclonal gammopathy.5 Histologically, Garner at al review many of the same lesions noted in this particular case, however their study identified inflammation involving the adventitial and medial tissue next of small veins and venules5, which was not particularly prominent in this slide. The EM findings in this study5 are similar to those noted by the contributor of this case, with intracytoplasmic virus particles found free within the cytoplasm of macrophages within the center of granulomas.
Careful inspection of the regions of the section in which granulomatous inflammation is not present (and in which the primary lesion is marked alveolar edema, will disclose the presence of numerous vacuolated histiocytes admixed with fewer lymphocytes in the alveoli immediately (<1mm) subjacent to the pleura. This is a characteristic incidental finding in ferrets, resulting from endogenous lipid pneumonia.
The composition of the JPC morphologic diagnosis above, due to multiple simultaneous pathogeneses in the submitted tissues, was a subject of spirited debate. The attribution of secondary inflammatory changes to a particular etiology was further complicated due to the history of prolonged therapeutic immunosuppression. The JPC tradition of assigning a separate morphologic diagnosis to each distinct process was ultimately upheld in the post-conference signout session.
Contributing Institution:
Massachusetts Institute of Technology,
16- 849, Division of Comparative Medicine
77 Massachusetts Ave,
Cambridge, MA 02139
https://web.mit.edu/comp-med/
References:
1. Addie DD. Feline coronavirus infections. In Greene CE, ed. (2012). Infectious Diseases of the Dog and Cat, 4th Edition. St Louis, MO, pp. 92-108.
2. Autieri CR, Miller CI, Scott KE, Kilgore A, Papscoe VA, Garner MM, Haupt JL, Bakthavatchalu V, Muthupalani S, Fox JG. Sysemic coronaviral disease in five ferrets. Comp Med 2015; 65(6):508-516.
3. Doria-Torra G, Viana B, Ramis A, Amarilla SP, Martinez J. Coronavirus infection in ferrets: antigen distribution and inflammatory response. Vet Pathol 2016, 53(6):1180-1186.
4. Fujii Y, Tochitani T, Kouchi M, Matsumoto, I, Yamada T, Funabashi H. Gloimeruloneprhtis in a ferret with feline coronavirus infection. J Vet Diagn Investig 2015;27(5):637-640
5. Garner MM, Ramsell K, Morera N, Juan-Sallés C, Jiménez J, Ardiaca M, Montesinos A, Teifke JP, Löhr CV, Evermann JF, Baszler TV, Nordhausen RW, Wise AG, Maes RK, Kiupel M. Clinicopathologic features of a systemic coronavirus-associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius). Vet Pathol 2008 45(2):236-46.
6. Li, TVC, Yoshizaki S, Kataoka M, Doan YH, Ami Y, Suzaki Y, Nakamura T, Takeda N, Wakita. Determination of ferret enteric coronavirus genome in laboratory ferrets. Emerg Inf Dis 2017; 23(9) 1568-1570
7. Lindemann DM, Eshar D, Schumacher LL, Almes KM, Rankin AJ. Pyogranulmatous panophthalmitis with systemic coronavirus diease in a domestic ferret (Mustela putorius furo). Vet Ophthalmol 2016; 19(2):167-171.
8. Martinez J, Ramis A, Reinacher M, Perpinan D. Detection of feline infectious peritonitis virus-like antigen in ferrets. Vet Record 2006: p. 523.
9. Michimae Y, Mikami S, Okimoto K, Toyosawa K, Matsumoto I, Kouchi M, Koujitani T, Inoue T, Seki T. The first case of feline infectious peritonitis-like pyogranuloma in a ferret infected by coronavirus in Japan. J Toxicol Pathol 2010; 23(2):99-101.
10. Minami S, Terada Y, Shimoda H, Takazawa M, Onuma M, Ota, A, Ota Y, Akabane Y, Tamukai K, Watanabe K, Naganuma Y, Kanagawa E, Nakamura K, Ohashi M, Takami Y, Miwa Y, Tanoue T, Ohwaki M, Ohta J, Une Y, Maeda, H. Establishment of serological test to detect antibody against ferret coronavirus. J Vet Med Sci 2016; 78(6):1013-1017.
11. Momberg-Jorgensen HC. Enzootic malignant granulomatosis in ferrets. Acta Pathol Microbiol Scand 1951;29:297-306.
12. Murray J, Kiupel M, Maes RK. Ferret coronavirus-associated diseases. Vet Clin North Am Exot Anim Pract 2010; 13(3):543-60.
13. Palley LS, Corning BF, Fox JG, Murphy JC, Gould DH. Parvovirus-associated syndrome (Aleutian disease) in two ferrets. J Am Vet Med Assoc 1992;201(1):100-6.
14. Porter HG, Porter DD, Larsen AE. Aleutian Disease in Ferrets. Infect Immun 1982; 36(1):379.
15. Williams BH, Kiupel M, West KH, Raymond JT, Grant CK, Glickman LT. Coronavirus- associated epizootic catarrhal enteritis in ferrets. J Am Assoc Vet Med 2000; 217(4):526-530.
16. Wise AG, Kiupel M, Garner MM, Clark AK, Maes RK. Comparative sequence analysis of the distal one-third of the genomes of a systemic and an enteric ferret coronavirus. Virus Res 2010; 149(1):42-50.
17. Wise AG, Kiupel M, Maes RK. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets. Virology 2006; 349:164-174.