Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 5
25 August 2019
CASE II: 160360 (JPC 4085120).
Signalment: Adult, female, European brown hare (Lepus europaeus).
History: Found dead in the woods
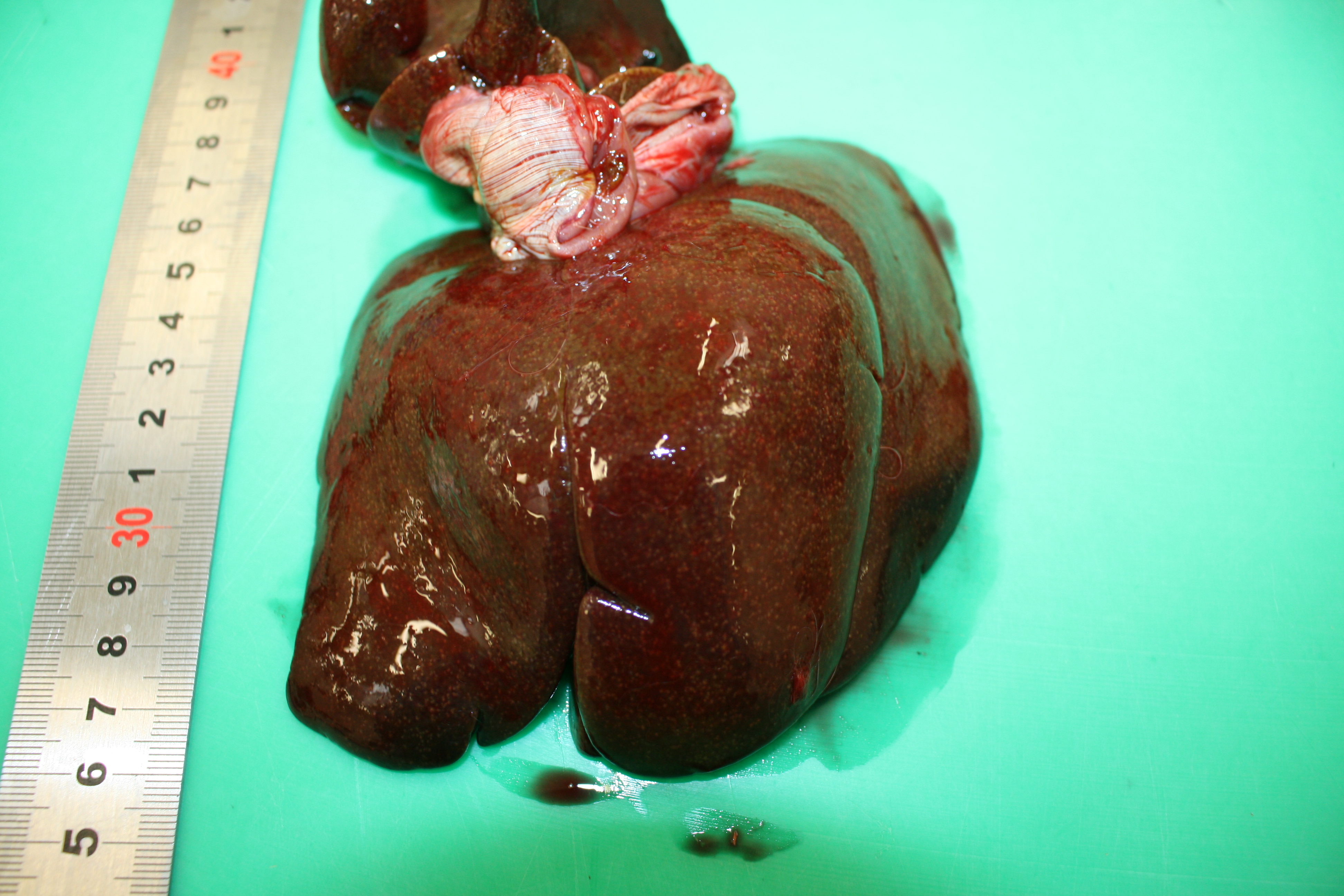
Gross Pathology: At necropsy the animal presented a severe jaundice. Mesenteric lymph nodes were enlarged and on cut section they presented multiple coalescing foci of caseous necrosis. The spleen was severely enlarged and was characterized by 1 mm white foci also present in the liver and the kidneys.
]
Laboratory results: Bacteriology : Yersinia pseudotuberculosis in liver and spleen.
Microscopic Description:
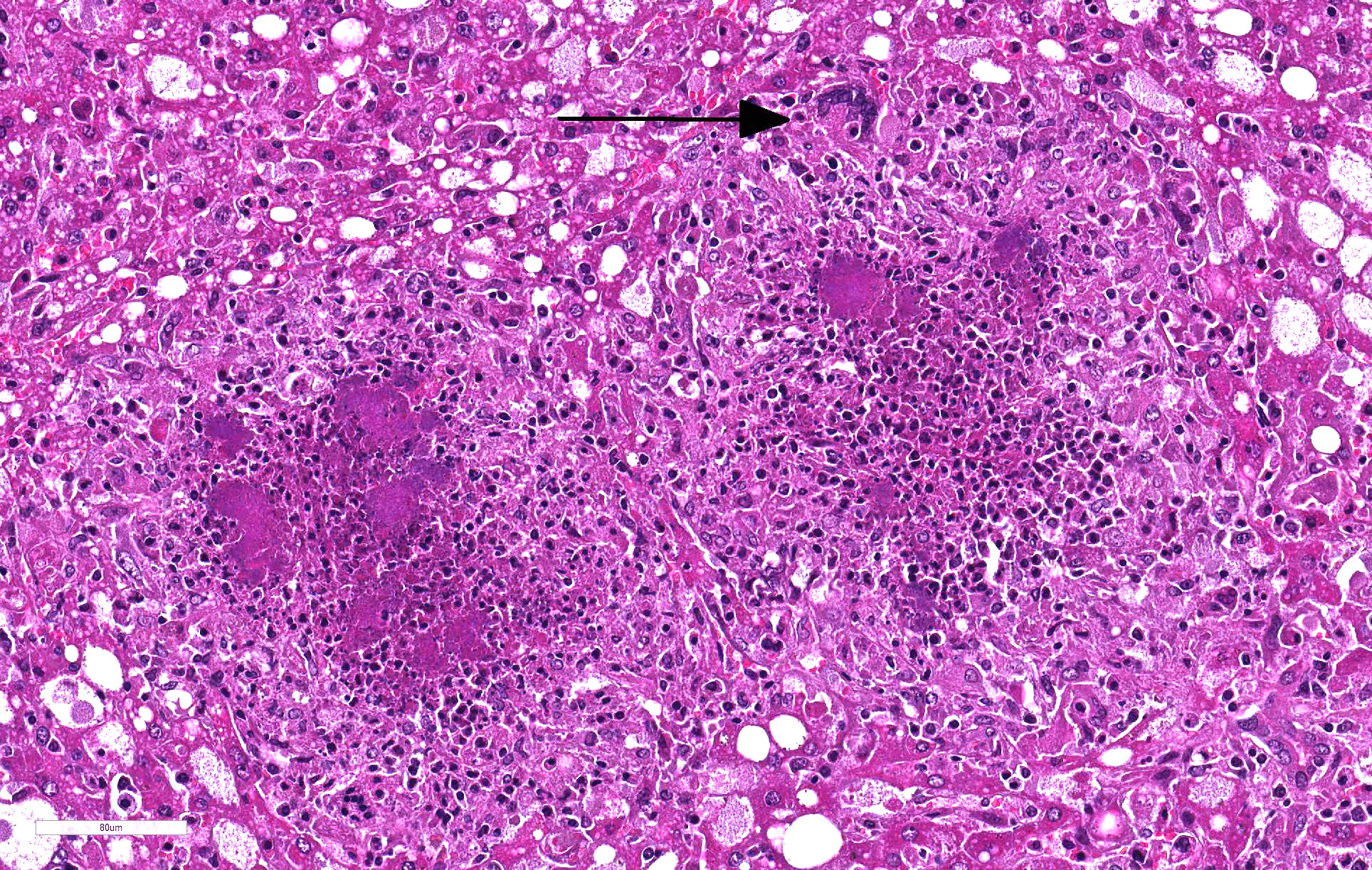
Liver: 60% of hepatic parenchyma is characterized by the presence of multifocal to coalescing, 1mm in size, randomly distributed inflammatory lesions (Fig. 4). These lesions are composed, from the periphery to the center, by a moderate amount of macrophages, epithelioid cells and less multinucleated giant cells (Langhans and foreign body types) admixed with scant heterophils. The inflammatory cells surround some cells (macrophages and hepatocytes) with condensed (pyknosis) or fragmented nuclei (karyorrhexis), nuclear and cellular debris (necrosis). The center of the lesion is occupied by small, 1µm in length, coccobacillar basophilic organisms (bacterial colonies) enmeshed within an eosinophilic material (fibrin). Liver parenchyma presents severe and diffuse hydropic degeneration, characterized by enlarged cells with clear granular eosinophilic cytoplasm (glycogenosis). A multifocal single cell necrosis is also present. Within the sinusoids a moderate amount of heterophils and monocytes are present, as well as Kupffer cells (Kupffer cell hyperplasia). An extramedullary hematopoiesis is evident within Disseâspace.
Contributor Morphologic
Diagnoses:
Liver: Hepatitis, granulomatous and necrotizing, multifocal to
coalescing, severe chronic with bacteria consistent with Yersinia
pseudotuberculosis.
Kidney (not submitted): Nephritis, granulomatous and necrotic, multifocal, chronic moderate with bacteria consistent with Yersinia pseudotuberculosis.
Spleen (not submitted): Splenitis, granulomatous and necrotic, multifocal to coalescing, chronic severe with ibacteria consistent with Yersinia pseudotuberculosis.
Contributor Comment: Yersinia pseudotuberculosis is a gram-negative, facultative intracellular coccobacillus, which occurs worldwide in wild rodents, lagomorphs and birds. Sporadic infections can occur in domestic animals and humans. It is shed in the feces by infected animals and it can survive and grow in the environment at low temperatures. Infection occurs by ingestion; the organism penetrates the intestinal mucosa through the M cells.3 Then it migrates to the Payer’s patches and by the lymphatic route to the mesenteric lymph nodes. If the organism becomes septicemic/bacteremic, it spreads to other organs, typically liver and spleen.
Pathogenic factors include:
- Invasin. This protein is expressed at low temperature conditions and it facilitates bacteria translocation through the intestinal epithelium into the lamina propria and Peyer’s patches. Invasin binds to β1 integrins on cells, favoring bacterial-cell adhesion.
- Type III secretion system (T3SS): it is a virulence mechanism found in several gram-negative bacteria. It is composed of a needle-like syringe that injects into the host cell the effector proteins. After being attached to host cells, Y. pseudotuberculosis uses this system to inject the Yersinia outer proteins (Yops) 3
- Yersinia outer proteins (Yops): there are 6 known Yops. These effector proteins alter the actin cytoskeletal structures to inhibit phagocytosis, induce apoptosis and downregulate proinflammatory responses. They are produced at 37°C environmental temperature, which means within the host.3
Gross typical lesions are white 1-10 mm foci in affected organs, mainly intestine, liver, spleen and mesenteric lymph nodes. In the large lesions a caseous necrosis is visible. Histologically, the lesions consist in micro abscesses composed of necrotic neutrophils with or without granulomatous reaction. Large bacterial colonies are easily visible.
In hares and rabbits differential diagnosis are Yersinia enterocolitica and Francisella tularensis. The three bacteria produce similar gross and histologic lesions and bacterial culture is necessary to identify them.
Contributing Institution:
Laboratoire d’Histopathologie animale, Vetagro Sup, campus veterinaire,
http://www.vetagro-sup.fr/
JPC Diagnosis: Liver: Hepatitis, necrotizing and granulomatous, multifocal to
coalescing, marked, with numerous large colonies of bacilli.
JPC Comment: The genus Yersinia is a member of the family Enterobacteriaceae and consists of 18 species of gram-negative bacilli, three of which, Y. enterocolitica, Y. pseudotuberculosis, and the causative agent of plague, Yersinia pestis are pathogenic for animals, Nonhuman primates and man are considered extremely susceptible to infection by these pathogens, although Y. enterocolitica (Yec) was not considered as a human or veterinary pathogen until the late 1960’s.1 In contrast, a number of domestic and wildlife species including pigs, rodents, and wild birds, are and important reservoirs of this pathogens and considered significantly less susceptible to their pathogenic effects. (Interestingly, pork chitterlings (intestine) have been often identified as a source of food-borne illness to young children, during the cleaning and preparation phase.)4 Y. enterocolitica has over 60 serotypes, but less than ten are pathogenic in primate hosts. It has six biotypes, of which one is highly pathogenic (1B), one is non-pathogenic (1A) and the remained, biotypes 2-5) are considered mildly pathogenic.2
The plasmid of Yersinia virulence (pYV) which encodes
for many of the virulence factors mentioned by the contributor is the most
important factor in pathogenicity. All biotypes are capable of mucosal
invasion; however only those strains with the pYV can migrate from Peyer’s
patches to mesenteric nodes, and onward to establish the necrotic lesions in
multiple organs demonstrated in this case. While this would logically mean
that identifying plasmids are an easy way to identify pathogenic serotypes, it
has been demonstrated that pathogenic bacteria may spontaneously lose this
plasmid when exposed to temperatures over 37C, prolonged storage, or frequent
passaging.2
The primary site of translocation in the intestine is via
the M cells. Some strains of Yec may cluster on M cells at a density of
over 1000X that of the surrounding absorptive villar epithelium.2 By
five days after infection, M cells and Peyer’s patches are usually destroyed2,
and the bacteria have migrated to mesenteric lymph nodes.
The contributor mentions several important virulence factors including the Type
III secretion system, a unique needle-like system which actively injects yersinial
outer proteins (Yops) into cells to aid in evasion of the immune response. A
few other virulence factors also bear mentioning: mucoid Yersiniae
factor, which closely resembles fimbriae of enterotoxigenic E. coli and
assists in mucosal attachment in early stages of infection, and several
heat-stable enterotoxins (which induce diarrhea in infected individuals).2
Yersinia enterocolitica has been a popular submission with seven cases in the last decade of the WSC alone (and over 25 since 1975). A list of more recent cases include: WSC 2018-2019 liver and spleen, African green monkey; WSC 2016-2017, Conf 8, case 4, lung, African Green monkey; WSC 2015-2016, Conf 12, Case 3 liver and cecum, hare; WSC 2013-2014, Conference 1, Case 3, small intestine and lymph node blackbuck; 2011-2012, WSC Conference 3, Case 1, Liver and spleen, guinea pig; and WSC 2010-2011, Conf 15, Case, 1, intestine, Indian macaque.
References:
1. Bakker J, Kondova I, de Groot CW Remarqe EJ, Heidt, PJ. A report on Yersinia-related mortality in a colony of new world monkeys. Lab Prim Newsletter. 2007; 46(3):11-15.
2. Bancerz-Lisiel A, Pieczywek, M, Lada P, Szweda W. The most important virulence markers of Yersinia enterocolitica and their role during infection. Genes (Basel) 2018; 9(5):pii: E234, doi: 10:3390/genes9050235
4. Sabina Y, Rahman A, Ray RC, Montet, D. Yersinia enterocolitica: Mode of transmission, molecular insights of virulence and pathogenesis of infection. J Path 2011; 1:1-10.