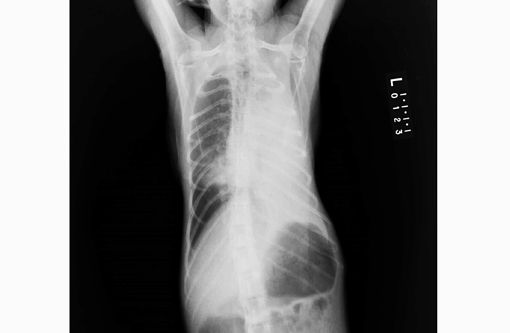
Signalment:
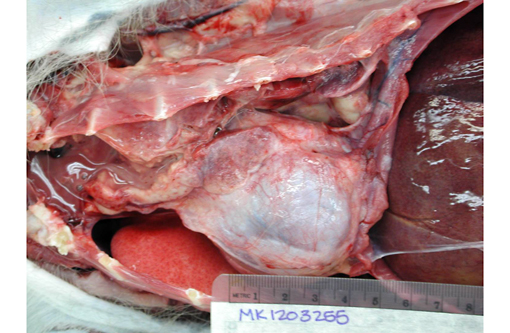
Gross Description:
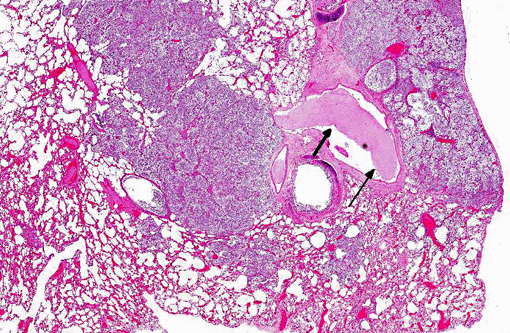
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
- Klebsiella pneumoniae was cultured from the pleural effusion, one of the nodules and a section of left lung lobe.
- Sections of lung and thoracic lymph nodes were negative for Mycobacteria sp. by PCR.
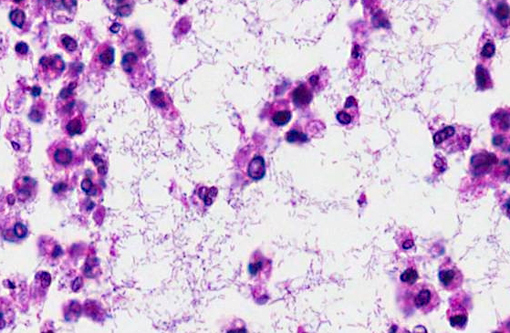
- Gram stain revealed numerous gram-negative rods.Â
- Fungal cysts, consistent with neumocystis spp., were not found with GMS stain.
- Mycobacteria spp. were not seen with Acid Fast stain.
Condition:
Contributor Comment:
Other lesions included:
- Pancreas islet cell hyalinization, multifocal, mild
- Heart, myocardial degeneration, loss and fibrosis, multifocal, minimal - mild
- Kidney, cyst, focal, mild
- Lymph node, lymphoid hyperplasia, moderate
Klebsiella pneumoniae is a gram-negative, non-spore-forming, facultative, anaerobic, nonmotile rod with a prominent capsule. Non-pathogenic strains can be found in soil, water, man and mammals; the bacteria colonize mucosal surfaces. Klebsiella pneumoniae afflicts the debilitated, immunosuppressed and those in hospitals and long-term care facilities and is the most frequent case of gram-negative pneumonia. Intravenous catheters and surgical sites may also serve as sources of infection.(4,10,13)
Disease in nonhuman primates may be associated with such stressors as shipping, quarantine and overcrowding. In New World monkeys and Old World monkeys, Klebsiella pneumoniae infection has been associated with septicemia, air sacculitis, pneumonia, pulmonary abscess, peritonitis, cystitis and meningitis. Vaccination with killed whole bacterin in owl monkeys, capsular polysaccharide in squirrel monkeys, and autogenous vaccines in marmosets have been used to reduce morbidity and mortality due to Klebsiella pneumoniae infection.(14)
Klebsiella pneumoniae in lab animals is a rare cause of enterotyphlitis, septicemia and necrotizing bronchopneumonia in rabbits; septicemia, bronchopneumonia, pericarditis, pleuritis and peritonitis in guinea pigs; lymph node and kidney abscesses as well as rhinitis in rats. In mice, cervical lymphadenopathy, liver and kidney abscesses, empyema, endo- and myocarditis and thrombosis are associated with, but not diagnostic for, Klebsiella pneumoniae infection.(6,7,8,9) In domestic animals, Klebsiella pneumoniae has been isolated from mares with endometritis and abortion and from foals with pneumonia and septicemia.(2,12)
A hypermucoviscous (HMV) variant of Klebsiella pneumoniae (HMV-KP) has been identified in humans and subsequently in animals. The virulence of HMV is thought to be due, in part, to capsular serotypes (K1 and K2) that carry genes MagA (mucoviscosity-associated gene/K1 specific capsular polymerase gene) and rmpA (regulator of mucoid phenotype) which make the bacteria more invasive and more resistant to phagocytosis.(13,15) Bacterial colonies appear mucoid and have a positive string test (an inoculation loop is pulled through the bacterial colony and the resulting string formed is greater than 5mm). In humans, HMV infection is unusual in that it infects healthy individuals and causes liver abscesses, pneumonia, meningitis, and endophthalmitis. The mode of infection is unknown but is thought to be via the intestine.(12,13)
Klebsiella pneumoniae with an HMV phenotype has been identified in African green monkeys in which abscesses were found in the abdomen, lungs, cerebellum and skin. The masses appeared to be centered on lymph nodes and there was associated peritonitis and adhesions to the intestines. Oral and rectal swabs of macaques in the same facility identified animals that were subclinically infected.(1,16)
Klebsiella pneumoniae HMV has been cultured from California sea lions dying shortly after being observed to be ill. Many of the animals were in good body condition but had bronchopneumonia, fibrinous pleuritis and abscess. Sea lions may serve as a potential source of zoonotic disease for swimmers.(5)
The HMV phenotype can be detected by rapid real-time PCR assays that target rmpA and magA genes. RAPD (rapid amplification of polymorphic DNA) can then be used to determine genetic variability between isolates.(3)
JPC Diagnosis:
Conference Comment:
The polysaccharide capsule is essential for Klebsiella virulence. It prevents phagocytosis, suppresses the activation of complement components (especially C3b), and may inhibit macrophage differentiation.(10) Klebsiella spp. have developed resistance to the host serum bactericidal effect, normally mediated by complement proteins. This resistance likely involves two factors: 1) capsular polysacchariade, which may mask LPS and prevent complement activation and 2) the structure of lipopolysaccharide (LPS). It is hypothesized that the O antigens of LPS extend through the capsule layer, where complement protein C3b is fixed preferentially to the longest O-polysaccharide side chains. This binding fixes C3b far enough away from the bacterial cell membrane that the formation of the lytic membrane attack complex (C5bC9) is prevented.(10) Pili, or fimbriae, are nonflagellar, filamentous bacterial surface projections that act as adhesions to bind host cells. Type 1 pili mediate bacterial colonization of mucus or epithelial cells of the urogenital, respiratory, and intestinal tracts, while type 3 pili adhere to endothelial cells, respiratory and urinary epithelium, tubular basement membranes, Bowmans capsules, and renal vessels.(10) Finally, since iron availability is a limiting factor for bacterial growth, Klebsiella spp. secrete siderophores, such as enterobactin and aerobactin. Siderophores are high-affinity, low-molecular-weight iron chelators that are capable of competitively taking up iron bound to host proteins.(10)
References:
2. Caswell JL, Williams KJ. The Respiratory System. In: Maxie MG ed., Jubb, Kennedy and Palmers Pathology of Domestic Animals, 5th ed., vol 2. Philadelphia, PA: Elsevier Limited; 2007: 632.
3. Hartman LJ, Selby EB, Whitehouse CA, Coyne SR, Jaissle JG, Twenhafel NA, Burke RL, and David A. Kulesh DA. Rapid Real-Time PCR Assays for Detection of Klebsiella pneumoniae with the rmpA or magA Genes Associated with the Hypermucoviscosity Phenotype - Screening of Nonhuman Primates. J Mol Diagn. 2009;11(5):464471.
4. Husain A, Kumar V. The Lung. In: Kumar V, Abbas AK, Fausto N, eds. Pathologic Basis of Disease, 7th ed. London, UK: Saunders/Elsevier; 2005: 748.
5. Jang S, Wheeler L, Carey RB, Jensen B, Crandall CM, Schrader KN, Jessup D, Colegrove K, Gulland FM. Pleuritis and suppurative pneumonia associated with a hypermucoviscosity phenotype of Klebsiella pneumoniae in California sea lions (Zalophus californianus).Vet Microbiol. 2010;141(1-2):174-177.
6. Percy DH, Barthold SW. The Guinea Pig. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed. Ames, IA: Blackwell Publishing; 2007: 222.
7. Percy DH, Barthold SW. The Mouse. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed. Ames, IA: Blackwell Publishing; 2007: 64.
8. Percy DH, Barthold SW. The Rabbit. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed. Ames, IA: Blackwell Publishing; 2007: 275.
9. Percy DH, Barthold SW. The Rat. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed. Ames, IA: Blackwell Publishing; 2007: 152.
10. Podschun R and Ullmann U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin Microbiol Rev. 1998;11(4):589-603.
11. Schmidt M, Zickler P, Hoffmann G, Haas S, Wissler M, Muessig A, Tisdale F, Kuramoto K, Andrews RG, Wu T, Kiem H-P, Dunbar CE, Von-Kalle C. Polyclonal long-term repopulating stem cell clones in a primate model. Blood. 2002;100(8): 2737-2743.
12. Shlafer DH, Miller RB. The Female Genital System Jubb, Kennedy and Palmers Pathology of Domestic Animals, 5th ed., vol 3. Philadelphia, PA: Elsevier Limited; 2007: 507.
13. Shon AS, Bajwa RP, Russ, TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107-118
14. Simmons J, Gibson S. Bacterial and Mycotic Diseases of Nonhuman Primates In: Abee CR, Mansfield K, Tardiff S, Morris T, eds. Nonhuman Primates in Biomedical Research, 2nd ed. London, UK: Elsevier; 2012: 128-130.
15. Soto E, LaMon V, Griffin M, Keirstead N, Palmour, R. Phenotypic and genotypic characterization of Klebsiella pneumoniae isolates recovered from nonhuman primates. J Wildl Dis. 2012;48(3):603-611.
16. Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE. Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae-identification of the hypermucoviscosity phenotype. Vet Pathol. 2008;45:226-231.