Signalment:
Gross Description:
Histopathologic Description:
Kidneys: Glomeruli are variably-sized and mostly hypocellular containing a high amount of eosinophilic, acellular and amorphous material (amyloid, Congo/Sirius red positive) and exhibit mesangial atrophy. Under UV light, the amyloid, in the Congo red stained sections, shows orange fluorescence (as described by Linke).(8) Congophilia is lost in sections pretreated with potassium permanganate confirming the amyloid to be of secondary origin. Amyloid is also seen in renal tubules, within the lumen of a pelvic artery (amyloid cast), and in the renal pelvis. Glomeruli also contain to lesser extent deposition of fibrous tissue and diffusely moderate to severely thickened Bowman capsules (Massons Trichrome stain positive). Mild to moderate, multifocal lymphoplasmacytic infiltration (variable within provided slides) and tubular mineralization (calcification) are present. Renal tubules often contain protein casts.
Arteries/arterioles: Moderate to severe deposition of amyloid and fibrosis are also seen in the intima and media of arteries and arterioles in the liver, left ventricle and septum.
Left adrenal gland: Adrenal medulla is densely cellular with cells arranged in packets and nests and supported by a moderately fine fibrovascular stroma that occasionally thickens and dissects the parenchyma, leading to a disarranged lobular pattern. The mass is composed of round to polygonal cells exhibiting mild to moderate anisocytosis and anisokaryosis. The cytoplasm of neoplastic cells is finely granular, eosinophilic and most often poorly demarcated. Nuclei are round to oval, hyperchromatic, with finely stippled chromatin and most frequently, no nucleoli. Mitotic figures are rare. Neoplastic cells are synaptophysin positive (neuroendocrine origin) and are found intravascularly within adjacent mesentery and extending directly from the adrenal gland into an adjacent large artery (phrenicoabdominal artery?), subcapsularly and supracapsularly (infiltrative growth pattern). Scattered binucleated cells, haemosiderophages and cytomegaly are observed. Occasional vascular degeneration with mineralization (calcification) is seen in the adrenal cortex. Due to the medullary mass, a moderate to severe, diffuse cortical atrophy is present. Transition between adrenal medulla and cortex is multifocally poorly demarcated and in scattered areas a rim of fibrous tissue separates the two regions (pseudocapsule formation).
Morphologic Diagnosis:
1- Kidney, severe diffuse glomerular, intratubular and pelvic amyloidosis, mild to moderate diffuse glomerulosclerosis and interstitial lymphoplasmacytic nephritis with focal calcification
2-Kidney, liver and heart, moderate to severe multifocal amyloid angiopathy and arterio/arteriolosclerosis
3-Adrenal, malignant pheochromocytoma with consecutive moderate to severe diffuse adrenal cortical atrophy
Condition:
Contributor Comment:
| Type of amyloid | Aetiology | Structures or organs mostly affected | Species and breeds most frequently affected |
| Primary (AL) or Immunoglobin derived - Localized or generalized | Immunoglobin λ or κ in plasma cells dyscrasias from B cell monoclonal
proliferation. 25 types identified | Spleen, heart, tongue, Kidneys, nerves and joints (atypical distribution) | Uncommon Most frequent in dogs, horses and cats |
| Secondary or reactive systemic amyloidosis | AA (amyloid associated) serum protein (an acute phase reactant and the major HDL apoliprotein) produced by hepatocytes after cytokine stimuli (mainly IL6) 5,10 | Kidneys, arteries, spleen, liver, enteric mucosa, joints (gallinae) | Dogs, cattle, horses and cats Less frequently: swine and goats |
| Familial | Peripheral nerves, kidneys, heart, liver | Cats: Abyssinian, Siamese, Oriental Dogs: Beagles, Sharpei, Gray collies, English foxhound. Cattle: Holstein (with bovine leukocyte adhesion deficiency) |
|
| Apoliprotein A1 derived | Apoliprotein A1 | Pulmonary arteries | Dogs |
| Islet amyloid polypeptide derived amyloid | Islet amyloid polypeptide Non-insulin dependent | Pancreas | Cats |
Pheochromocytomas are tumours of chromaffin cells and are the most frequent tumours observed in the adrenal medulla.2 Even though chromaffin cells produce catecolamines (epi/norepinephrine), in animals, clinical signs from their overproduction are rarely observed with a pheochromocytoma. When present, these symptoms consist mostly in tachycardia, cardiac hypertrophy and arterial sclerosis. In the rat, epinephrine has also been referred to act as a stimulant of interleukin release and secondary acute phase reactant.(7) Pheochromocytoma pathogenesis is unclear and has been associated in rats with chronic progressive glomerulopathy.(2,14,16) Genetic factors, pituitary tumours, hyperthyroidism, autonomic nervous system stimulation, hypercalcaemia, vitamin D3 and diets rich in calcium, retinoids or sugars have also been implicated.(2,14,16,23) Interaction between the kidney and the adrenal gland include renalase, which is produced in the glomeruli and proximal tubules and induces metabolisation of catecholamines, and thus lead to a decrease in the blood pressure.(3,7,19) There is a reduction in renalase synthesis in uraemic and end stage renal disease patients.(3,8,19) The renalase gene is also expressed in the heart, skeletal muscle and liver.(8) In animals, hypertension is most commonly of secondary nature and associated with chronic renal failure. However, the reverse can happen, where renal changes can also be due to reduction in renal perfusion secondary to hypertension.(13)
Arteriolosclerosis includes various pressure-induced vascular changes and can be either mainly hyaline or hypertrophic. In the uraemic dog, the arterial and arteriolar lesions consist of deposition of subendothelial fibrin, internal elastic lamina dystrophy, necrosis of smooth muscle, mineralization and occasionally neutrophilic infiltration.(13) All of these changes were seen in this case together with the amyloid angiopathy, leading to the hypothesis that together with glomerulosclerosis, arteriolosclerosis was a more predominant feature that worsened and no longer is presented as blatant lesions.
Secondary hyperparathyroidism is seen in chronic renal failure and leads to osseous resorption in an attempt to increase the calcium levels. This mechanism changes the Ca:Ph ratio and as a consequence dystrophic calcification occurs.
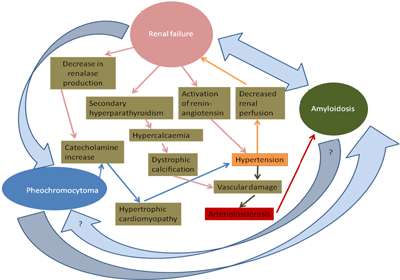
The conditions seen in this case and their severity and distribution suggest that they are aetiologically related. Below, based on the above provided information, a hypothetical graphical simplifying attempt of the relationship between the present conditions is provided.
JPC Diagnosis:
1. Adrenal gland: Malignant pheochromocytoma.
2. Kidney: Amyloid, glomerular, segmental to global, diffuse, moderate, with multifocal lymphoplasmacytic interstitial nephritis.
3. Heart, mural arteries: Arteriosclerosis, hyaline, multifocal, mild (hyalinosis), with cardiac myofiber loss and fibrosis.
Conference Comment:
Primary amyloidosis is rare in animals and may be encountered in the horse in the nasal vestibule and rostral parts of the nasal septum and turbinates. Secondary amyloidosis is far more common due to a reactive change caused by the synthesis of serum amyloid A (SAA) in the liver in response to IL-1, IL-6, and TNF in chronic inflammation. Cheetahs and Siberian tigers develop renal medullary interstitial amyloidosis in response to gastritis. Familial amyloidosis is usually autosomal recessive, and autosomal dominant in Abyssinian cats, which along with the Chinese Shar Pei develop amyloid in the kidneys, and Siamese cats develop amyloid in the liver. Localized amyloidosis can be deposited in the pancreas of cats and non-human primates with type II diabetes mellitus. Chickens can develop amyloid arthropathy associated with Enterococcus faecalis, and common sites of amyloid in geese and swans are the spleen and kidneys. In dogs, Hepatozoon americium and Ehrlichia can is have been associated with renal amyloidosis and glomerulopathy, and excess dietary vitamin A has been associated with renal amyloidosis in cats(12,13,19,20,22) .
Renal amyloidosis may be presumptively diagnosed from a urine protein:creatinine of greater than 18. A ratio of less than 0.5 is considered normal, 1-3 indicates tubular disease, and greater than 3 indicates glomerular disease. Ultrastructurally, amyloid appears as non-branching fibrils of 0.7-1.0 um diameter that form single to laterallyaggregated bundles or interlocking mesh-like ribbons that lack periodicity(6).
A differential diagnosis for the mural thickening of coronary arteries in this case is hyalinosis, a relatively common finding in the older dog. Hypertrophic hyalinization (hyalinosis) generally occurs in the intramural coronary and small meningeal and cerebral arteries of old dogs. There is generally no clinical significance in the CNS lesions, but this may result in multifocal intramural myocardial infarction. If valvular endocardiosis is present, these two lesions may lead to congestive heart failure. The mural deposits are most often fibrin or glycosaminoglycans (GAGs). Amyloid, as seen in this case(13) is far less common in hyalinosis.
Vascular invasion in the adrenal gland was not present on all slides.
References:
2. Capen C: Endocrine glands. In: Pathology of Domestic Animals, ed. MG M, pp. 419-422. Saunders Elsevier, 2007
3. Desir GV: Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr Opin Nephrol Hypertens 17: 181-185, 2008
4. Findeisen P, Zapatka M, Peccerella T, Matzk H, Neumaier M, Schadendorf D, Ugurel S: Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol 27: 2199-2208, 2009
5. Gruys E: Protein folding pathology in domestic animals. J Zhejiang Univ Sci 5: 1226-1238, 2004 24. Gueft B, Ghidoni JJ. The Site of Formation and Ultrastructure of Amyloid. Am J Pathol. 1963 November; 43(5): 837854.
6. Kahl M, Schade R: Catecholaminergic modulation of rat acute phase reactants. Agents Actions 32: 98-99, 1991
7. Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV: Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation 117: 1277-1282, 2008
8. Linke RP: Highly sensitive diagnosis of amyloid and various amyloid syndromes using Congo red fluorescence. Virchows Arch 436: 439-448, 2000
9. Malle E, Sodin-Semrl S, Kovacevic A: Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci 66: 9-26, 2009
10. Maxie MG NS: Urinary system. In: Pathology of Domestic Animals, ed. MG M, 5th edition ed., pp. 463-465. Saunders Elsevier, 2007
11. Maxie MN, SJ: Urinary system. In: Pathology of Domestic Animals, ed. MG M, 5th edition ed., pp. 463-465. Saunders Elsevier, 2007
12. Maxie MR, WF: Cardiovascular system. In: Pathology of Domestic Animals, ed. MG M, 5th edition ed., pp. 59- 60. Saunders Elsevier, 2007
13. Newman SJ. The urinary system. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. St. Louis, MO: Elsevier; 2012:627-628,640.
14. Nyska A, Haseman JK, Hailey JR, Smetana S, Maronpot RR: The association between severe nephropathy and pheochromocytoma in the male F344 rat -- the National Toxicology Program experience. Toxicol Pathol 27: 456- 462, 1999
15. Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Loening SA, Jung K: Serum amyloid A as indicator of distant metastases but not as early tumor marker in patients with renal cell carcinoma. Cancer Lett 269: 85-92, 2008
16. Rosol TJ, Yarrington JT, Latendresse J, Capen CC: Adrenal gland: structure, function, and mechanisms of toxicity. Toxicol Pathol 29: 41-48, 2001
17. Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: effects on the cardiovascular system. Circulation 116: 85-97, 2007
18. Snyder PW. Diseases of immunity. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. St. Louis, MO: Elsevier; 2012:284-288.
19. Stalker MJ HM: Liver and biliary system. In: Pathology of Domestic Animals, ed. MG M, 5th edition ed., pp. 315-316. Saunders Elsevier, 2007
20. Steinhoff MM, Wells SA, Jr., DeSchryver-Kecskemeti K: Stromal amyloid in pheochromocytomas. Hum Pathol 23: 33-36, 1992
21. Tecles F, Caldin M, Zanella A, Membiela F, Tvarijonaviciute A, Subiela SM, Ceron JJ: Serum acute phase protein concentrations in female dogs with mammary tumors. J Vet Diagn Invest 21: 214-219, 2009
22. Terio KA, OBrien T, Lamberski N, Famula TR, Munson L. Amyloidosis in black-footed cats (Felis nigripes). Vet Pathol. 2008;43(3):393-400.
23. Tischler AS, Powers JF, Alroy J: Animal models of pheochromocytoma. Histol Histopathol 19: 883-895, 2004