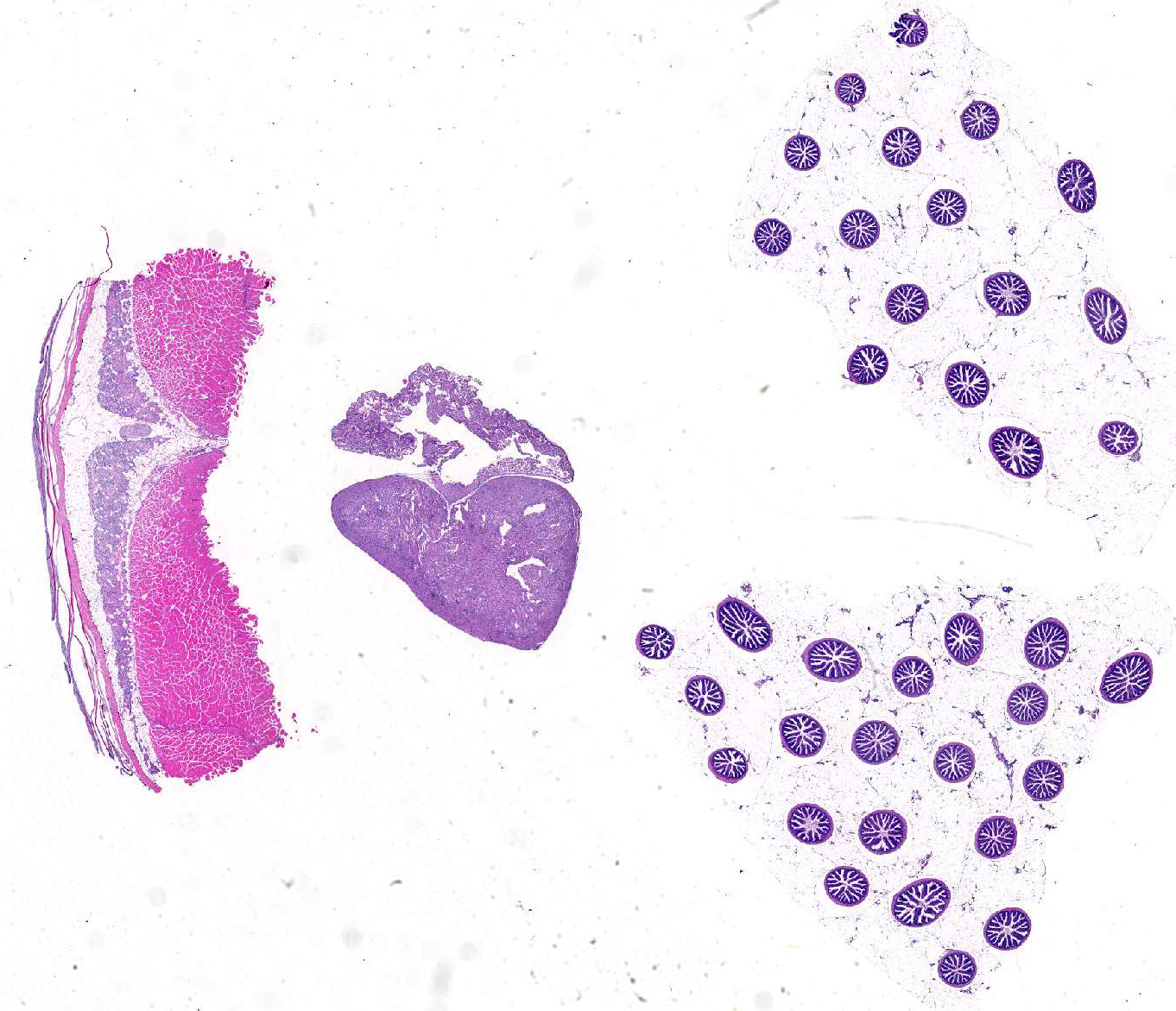
Signalment:
Gross Description:
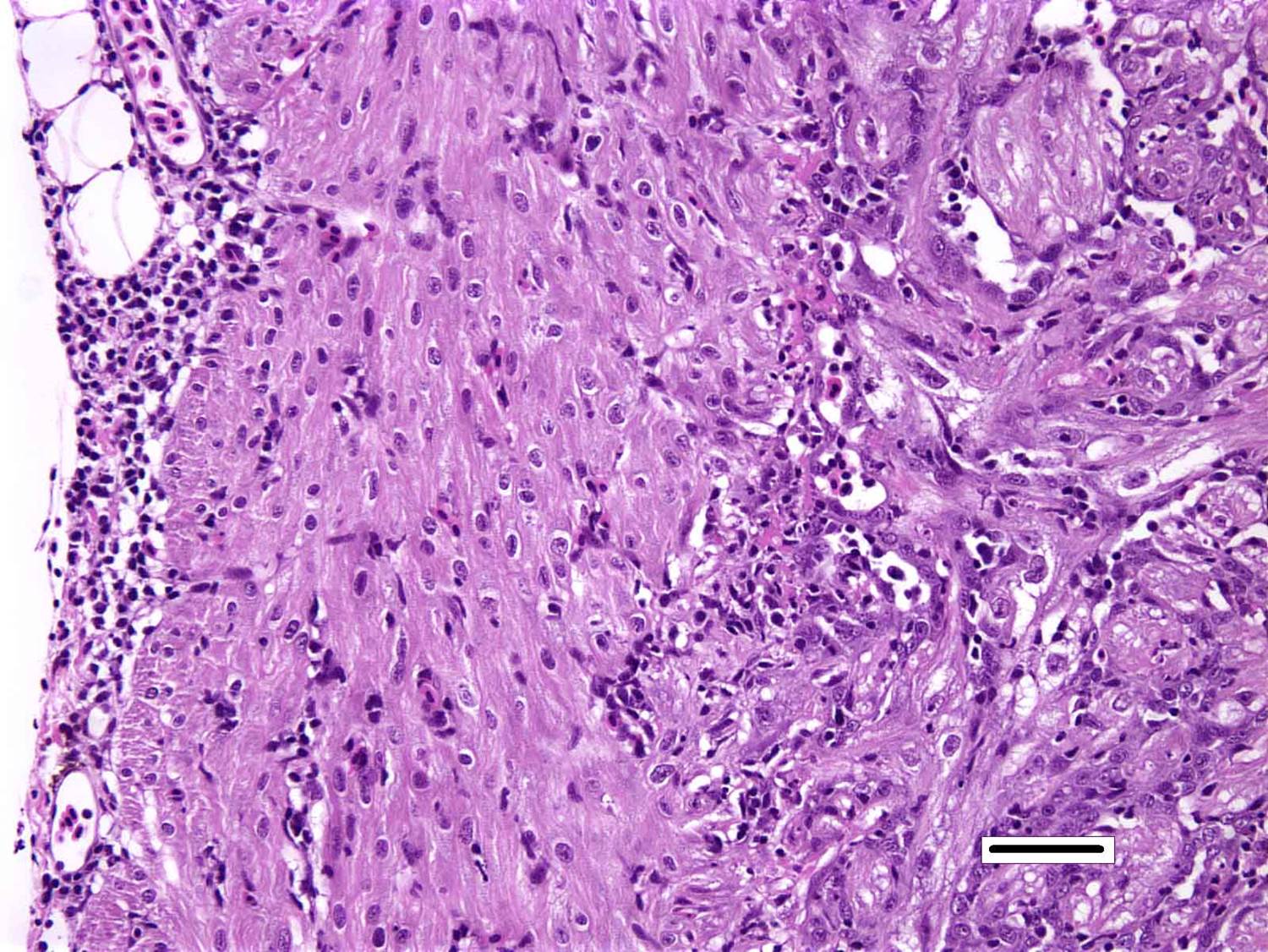
Histopathologic Description:
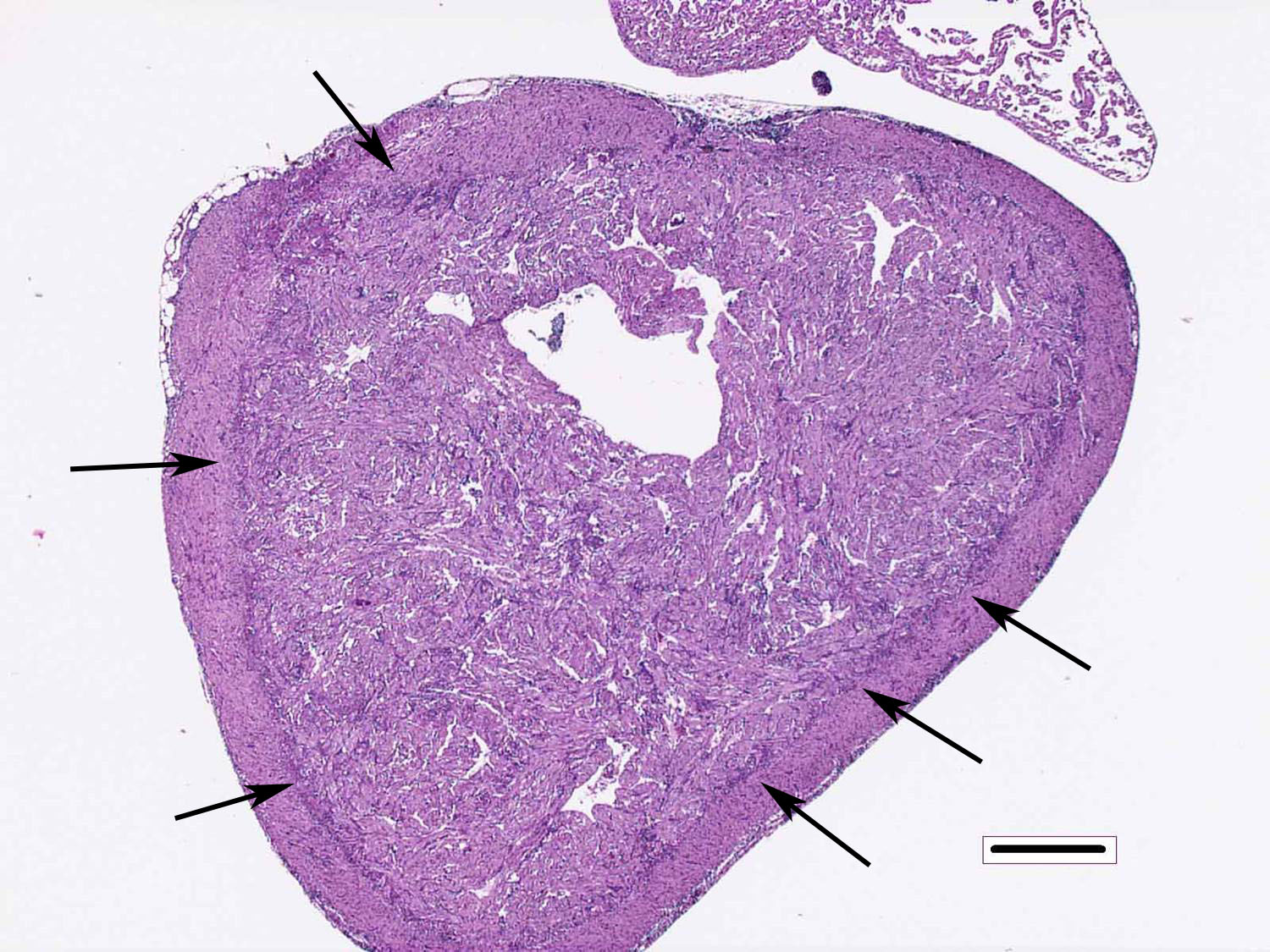
Heart: There are degenerative changes of the cardiomyocytes in the spongy and compact layers of the ventricle, characterized by marked cytoplasmic hypereosinophilia, with variable vacuolation and occasional shrunken nuclei. In these areas, there are low to moderate numbers of lymphocytes and macrophages. Multifocally, there is increased cellularity, prominence and occasional karyomegaly of endocardial endothelium (hyperplasia). Multifocally, the epicardium is expanded by mild to moderate numbers of lymphocytes and macrophages.
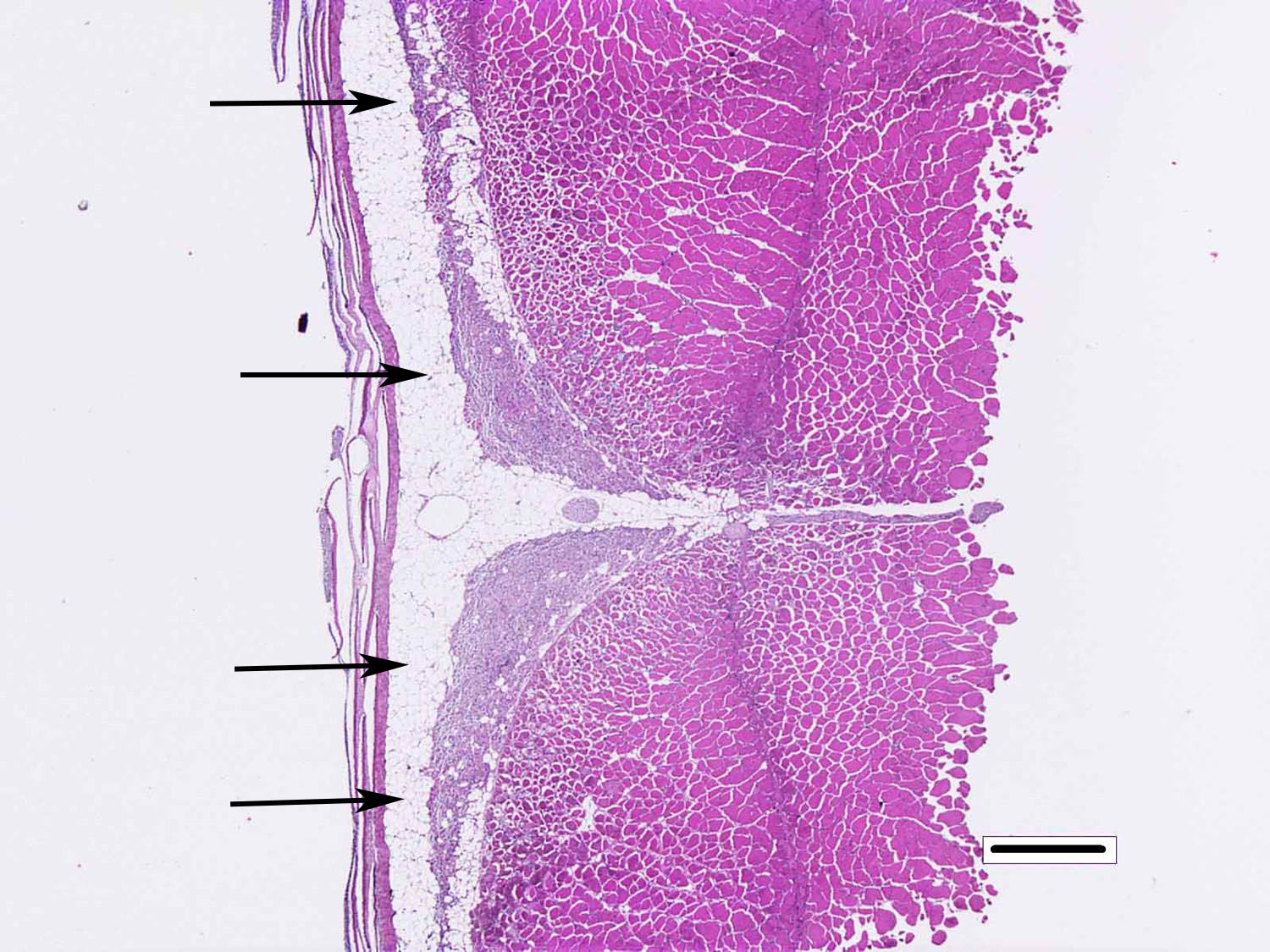
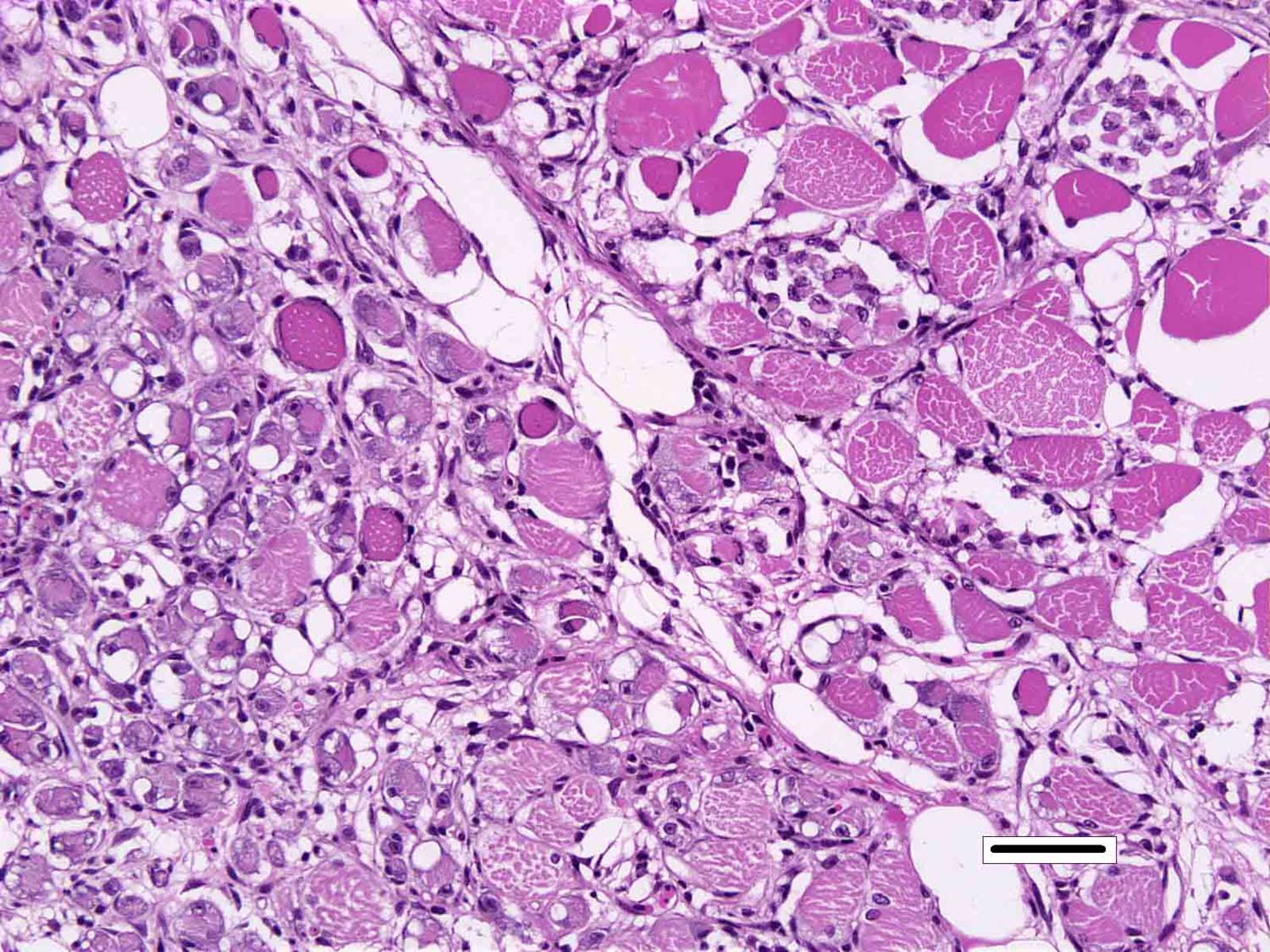
Skeletal muscle: Multifocally, there is marked loss of myofibres in the red muscle. The majority of the remaining myofibres are shrunken with densely eosinophilic sarcoplasm, surrounded by moderate numbers of mononuclear inflammatory cells.� In the white muscle, there are scattered necrotic and degenerating myofibres, with swollen fragmented, eosinophilic sarcoplasm, central migration of nuclei, and sarcoplasmic infiltration by macrophages. Affected myofibres are surrounded by low numbers of lymphocytes and macrophages.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
At present, six closely related subtypes have been identified for SAV4,5, which differ in host specificity, geographical location7,10 and aquatic environments (Table 1). SAV-2 is the only subtype commonly detected in freshwater systems, causing sleeping disease in freshwater trout. Current research also suggests there may be differences between strains in the infection dynamics7 as well as minor differences in prevalence and severity of the tissue damage8. Naturally occurring outbreaks of PD in farmed Atlantic salmon have only been reported in the seawater phase of production.15 To date, there has been no evidence of vertical transmission of the disease11 with horizontal transmission being by far the most important means of spreading the virus1,19, with shedding of mucus and feces described as transmission routes for SAV.
Table 1. Summary of Salmonid alphavirus subtypes, their geographical distribution and species susceptibility
|
SAV Subtype |
Species |
Production Phase |
Country |
|
SAV-1 |
Atlantic salmon (Salmo salar) |
Seawater |
Ireland and Scotland |
|
SAV-2 |
Rainbow trout (Oncorhynchus mykiss) |
Freshwater |
France, England, Scotland, Spain, Croatia and Germany |
|
SAV-2 (Marine) |
Atlantic salmon (Salmo salar) |
Seawater |
Scotland and Norway |
|
SAV-3 |
Atlantic salmon (Salmo salar) Rainbow trout (Oncorhynchus mykiss) |
Seawater |
Norway |
|
SAV-4 |
Atlantic salmon (Salmo salar) |
Seawater |
Ireland and Scotland |
|
SAV-5 |
Atlantic salmon (Salmo salar) |
Seawater |
Scotland |
|
SAV-6 |
Atlantic salmon (Salmo salar) |
Seawater |
Ireland |
JPC Diagnosis:
2. Heart, ventricle: Epicarditis and myocarditis, lymphocytic, diffuse, moderate with multifocal mild myocardiocyte necrosis.
3. Skeletal muscle, red: Degeneration and necrosis, multifocal to coalescing, severe with histiocytic myositis.
4. �Skeletal muscle, white: Degeneration and necrosis, multifocal to coalescing, mild to moderate with histiocytic myositis.
Conference Comment:
Both pancreas disease (PD) of salmon, seen in this case, and sleeping disease (SD) of rainbow trout is caused by related SAV infection. This virus is in the genus Alphavirus and family Togaviridae, a group of important enveloped ssRNA viruses.9,15 Other members of the Togaviridae family include eastern, western, and Venezuelan equine encephalitis viruses. Generally, this family of viruses is associated with transmission by insects, usually mosquitoes. An aquatic arthropod vector has not yet been identified for SAV; however, direct horizontal transmission has been well documented.9,15 The virus can survive for extended periods of time in the water outside of the host with a marked increase in survival in sea water compared to fresh water. The virus can also survive for long periods within the fat of dead fish and leaked fat droplets floating on the surface may contribute to long distance spread of the virus. Vertical transmission has not been shown to be a significant route of infection.9,15
References:
1.     Bratland A, Nylund A. Studies on the possibility of vertical transmission of Norwegian salmonid alphavirus in production of Atlantic salmon in Norway. J Aquat Anim Health. 2009; 21:173�178.
2.     Christie KE, Graham DA, McLoughlin MF, Villoing S, Todd D, Knappskog D. Experimental infection of Atlantic salmon Salmo salar pre-smolts by IP injection with new Irish and Norwegian salmonid alphavirus (SAV) isolates: a comparative study. Dis Aquat Organ. 2007; 75:13�22.
3.     Ferguson HW, Poppe TT, and Speare DJ. Cardiomyopathy in farmed Norwegian salmon. Dis Aquat Organ. 1990; 8:225�231.
4.     Fringuelli E, Rowley HM, Wilson JC, Hunter R, Rodger H, Graham DA. Phylogenetic analyses and molecular epidemiology of European salmonid alphaviruses (SAV) based on partial E2 and nsP3 gene nucleotide sequences. J Fish Dis. 2008; 31:811�23.
5.     Graham DA, Fringuelli E, Wilson C, Rowley HM, Brown A, Rodger H, et al. Prospective longitudinal studies of salmonid alphavirus infections on two Atlantic salmon farms in Ireland; evidence for viral persistence. J Fish Dis. 2010; 33(2):123�35.
6.     Graham DA, Fringuelli E, Rowley HM, Cockerill D, Cox DI, Turnbull T, et al. Geographical distribution of salmonid alphavirus subtypes in marine farmed Atlantic salmon, Salmo salar L., in Scotland and Ireland. J Fish Dis. 2012; 35:755�65.
7.     Graham DA, Frost P, McLaughlin K, Rowley HM, Gabestad I, Gordon A, et al. A comparative study of marine salmonid alphavirus subtypes 1�6 using an experimental cohabitation challenge model. J Fish Dis. 2011; 34:273�86.
8.     Haugland �, Mikalsen AB, Nilsen P, et al. Cardiomyopathy syndrome of Atlantic salmon (Salmo salar L.) is caused by a double-stranded RNA virus of the Totiviridae family. J Virol. 2011; 85(11):5275�5286.
9.     Herath TK, Ferguson HW, et al. Pathogenesis of experimental salmonid alphavirus infection in vivo: An ultrastructural insight. Vet Res. 2016; 47:7-18.
10. Jansen MD, Taksdal T, Wasmuth MA, Gjerset B, Brun E, Olsen AB, et al. Salmonid alphavirus (SAV) and pancreas disease (PD) in Atlantic salmon, Salmo salar L., in freshwater and seawater sites in Norway from 2006 to 2008. J Fish Dis. 2010; 33:391�402.
11. Kongtorp RT, Halse M, Taksdal T, and Falk K. Longitudinal study of a natural outbreak of heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L. J Fish Dis. 2006; 29(4):233�244.
12. Kongtorp RT, Sten A, Andreassen PA, Aspehaug V, Graham DA, Lyngstad TM, et al. Lack of evidence for vertical transmission of SAV 3 using gametes of Atlantic salmon, Salmo salar L., exposed by natural and experimental routes. J Fish Dis. 2010; 33:879�88.
13. Kongtorp RT, Taksdal T, and Lyng�y A. Pathology of heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 2004; 59(3):217�224.
14. L�voll M, Wiik-Nielsen J, Grove S, et al. A novel totivirus and piscine reovirus (PRV) in Atlantic salmon (Salmo salar) with cardiomyopathy syndrome (CMS). Virol J. 2010; 7:309.
15. McLoughlin M, Graham D. Alphavirus infections in salmonids �a review. J Fish Dis. 2007; 30:511�31.
16. Munro ALS, Ellis EAE, McVicar AH, McLay HA, Needham EA. An exocrine pancreas disease of farmed Atlantic salmon in Scotland. Helgol Meeresun. 1984; 37:571�86.
17. Palacios G, L�voll M, Tengs T, et al. Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS One. 2010; 5(7):e11487.
18. Poppe TT, and Ferguson HW. Cardiovascular system. In: Ferguson HW, ed. Systemic Pathology of Fish: A Text and Atlas of Normal Tissue Responses in Teleosts, and Their Responses in Disease. 2nd ed. London, UK: Scotian Press, 2006:141�167.
19. Rodger H, Mitchell S. Epidemiological observations of pancreas disease of farmed Atlantic salmon, Salmo salar L., in Ireland. J Fish Dis. 2007; 30:157�67.
20. Rodger HD, Murphy TM, Drinan EM, & Rice DA. Acute skeletal myopathy in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 1991; 12:17�23.
21. Wiik�Nielsen CR, Ski PMR, Aunsmo A, and L�voll M. Prevalence of viral RNA from piscine reovirus and piscine myocarditis virus in Atlantic salmon, Salmo salar L., broodfish and progeny. J Fish Dis. 2012; 35:169�171.