CASE 2: 19F156892 (JPC 4155173-00)
Signalment:
14-year-old female spayed domestic short haired cat (Felis catus)
History:
The patient presented with complaints of progressive paralysis in the hind limbs and draining skin lesions of the hind feet and face. The patient had been examined and treated unsuccessfully by veterinarians at three other clinics over the last several months. The patient was an indoor-only cat, was current on vaccinations, was fed a commercial dry cat food, and had moved with her owners to Wyoming from North Carolina.
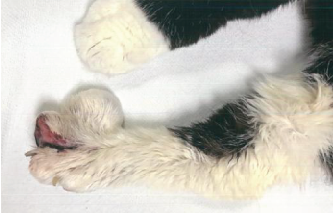
On physical exam, the patient was thin (3.08 kg) and somewhat depressed but responsive to handling. She was unable to stand on her hind limbs and preferred to rest on her elbows and forearms rather than stand on her front feet. She was afebrile (temp = 100.3 F). Withdrawal and deep pain reflexes were present in both hind legs, but her tail had reduced sensation and her bladder was large and easily expressed. Anal tone was judged to be somewhat reduced. Anisocoria (L > R) was noted. Very firm, nodular swellings of all the digits of the hind feet were present, and the ends of several of the toes were deeply ulcerated and matted with blood and purulent-type discharge. Similar, less dramatic nodular ulcers were present in the front feet and also involved the right upper lip. Additionally, hard, bony masses could be palpated on the left 5th rib and the distal right antebrachium and metatarsal regions. Increased bronchovesicular pulmonary sounds were ausculted diffusely but were particularly prominent in the right cranial and dorsocaudal quadrants. The initial differential diagnostic list included pulmonary hypertrophic osteopathy, metastatic neoplasia, and systemic mycotic disease (including sporotrichosis).
Gross Pathology:
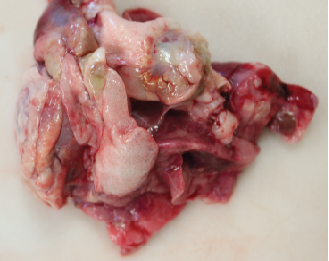
A 14-year-old female spayed domestic short haired cat weighing 3.08 kg was presented for necropsy in excellent postmortem condition with minimal autolysis. The cat was in thin body condition with mildly reduced stores of visceral and subcutaneous adipose tissue. On external exam, there was an open wound with a fistulous draining tract in the skin overlying the right maxillary canine, extending upward to the lateral aspect of the right nostril. The wound was characterized by a peripheral rim of chronic hemorrhage with a central tract extending through the subcutis and the underlying musculature. There was loss of the underlying maxillary bone and rostrum, with dark discoloration and loosening of the right maxillary canine in the alveolar socket. The distal aspects of multiple digits on both hindlimbs had similar external wounds with surface ulceration and deep penetrating tracts. The front limbs had less severe ulcerations on the digital surfaces at the junction of the haired skin and the paw pad. Open lesions oozed serous fluid and/or small amounts of purulent exudate. The phalanges were expanded up to 10 times the normal diameter by irregular, circumferential bony proliferations. Additionally, there was similar bony expansion of the right metatarsal bones and the left tibial metaphysis. The mid-bodies of ribs 5 through 7 on the left side of the thorax were focally disrupted by a circular bony lesion with central cavitation.
The thorax contained approximately 10mL of serosanguineous free fluid. All lung lobes were multifocally disrupted by discrete to coalescing nodules that were pale tan to white, solid to cystic or cavitated, and occasionally oozed purulent to mucinous material on cut section. Approximately 60% of the lung parenchyma was affected, and nodules ranged in size from ~3mm to 3cm. Small nodules on the pleural surface had an umbilicated appearance with central depressions and peripheral rims of white discoloration. There was emphysematous change at the borders of the lung lobes. The tracheobronchial lymph nodes were expanded up to 20 times normal and were completely effaced by similar solid to cystic nodules.
Bilaterally, the kidneys had multifocal, irregularly shaped pitted depressions on capsular surface that extended deep into the cortical parenchyma (consistent with chronic infarcts). The liver was diffusely congested and moderately firm. The stomach and small intestines were empty. The distal colon was moderately dilated and contained a large amount of formed feces.
Laboratory results:
Chemistry Panel
|
Test |
Result |
Normal Range |
High or Low Units |
|
Total Protein |
9.5 |
5.7 - 8.0 |
>range g/dl |
|
Albumin |
4.7 |
2.4 - 3.8 |
>range g/dl |
|
Globulin |
4.8 |
2.4 - 4.7 |
>range g/dl |
|
A/G Ratio |
1 |
0.6 - 1.1 |
in range |
|
ALP |
21 |
12.0 - 65.0 |
in range U/l |
|
ALT (SGPT) |
20 |
8.3 - 53.0 |
in range U/l |
|
Bilirubin, total |
4.4 |
0.1 - 0.5 |
>range mg/dl |
|
Glucose |
120 |
61 - 124 |
in range mg/dl |
|
Cholesterol |
160 |
71.0 - 161.0 |
in range mg/dl |
|
Amylase |
322 |
371 - 1193.0 |
|
|
Lipase |
< 10 |
0.0 ? 76 |
in range U/l |
|
Creatinine |
0.5 |
0.5 - 1.9 |
in range mg/dl |
|
BUN |
15.4 |
15.0 - 31.1 |
in range mg/dl |
|
Sodium |
> 250 |
146.0 - 159.0 |
>range meq/l |
|
Chloride |
> 175 |
108.0 - 130.0 |
>range meq/l |
|
Potassium |
2.5 |
3.8 - 5.3 |
|
|
Calcium |
7.5 |
7.9 - 10.9 |
|
|
Phos |
5.5 |
4.0 - 7.3 |
in range mg/dl |
|
TCO2 |
33.1 |
16.0 - 22 |
>range |
|
Anion Gap |
**** |
7 - 17 |
|
|
Hemolysis Moderate |
|||
CBC
|
Test |
Result |
Normal Range |
High or Low Units |
|
WBC Count, Manual |
3.6 |
5.5 - 19.5 |
|
|
Bands |
6 |
0 - 3 |
>range % |
|
Neutrophils |
75 |
35 - 75 |
in range % |
|
Lymphocytes |
14 |
20 - 55 |
|
|
Monocytes |
4 |
1 ? 4 |
in range % |
|
Eosinophils |
1 |
2 - 12 |
|
|
Basophils |
0 |
0 - 0.5 |
in range % |
|
Platelets |
109 |
300 - 700 |
|
|
Mild Platelet Clumping |
|
|
|
|
RBC Count |
4.93 |
5.0 - 10.0 |
|
|
PCV |
18.7 |
30.0 - 45.0 |
|
|
Hemoglobin |
5.8 |
8.0 - 15.0 |
|
|
MCV |
37.8 |
39.0 - 55.0 |
|
|
MCHC |
31.0 |
30.0 - 36.0 |
in range g/dl |
|
MCH |
11.7 |
13.0 - 17.0 |
|
|
RDW |
19.7 |
14.0 - 19.0 |
>range %. |
Microscopic description:
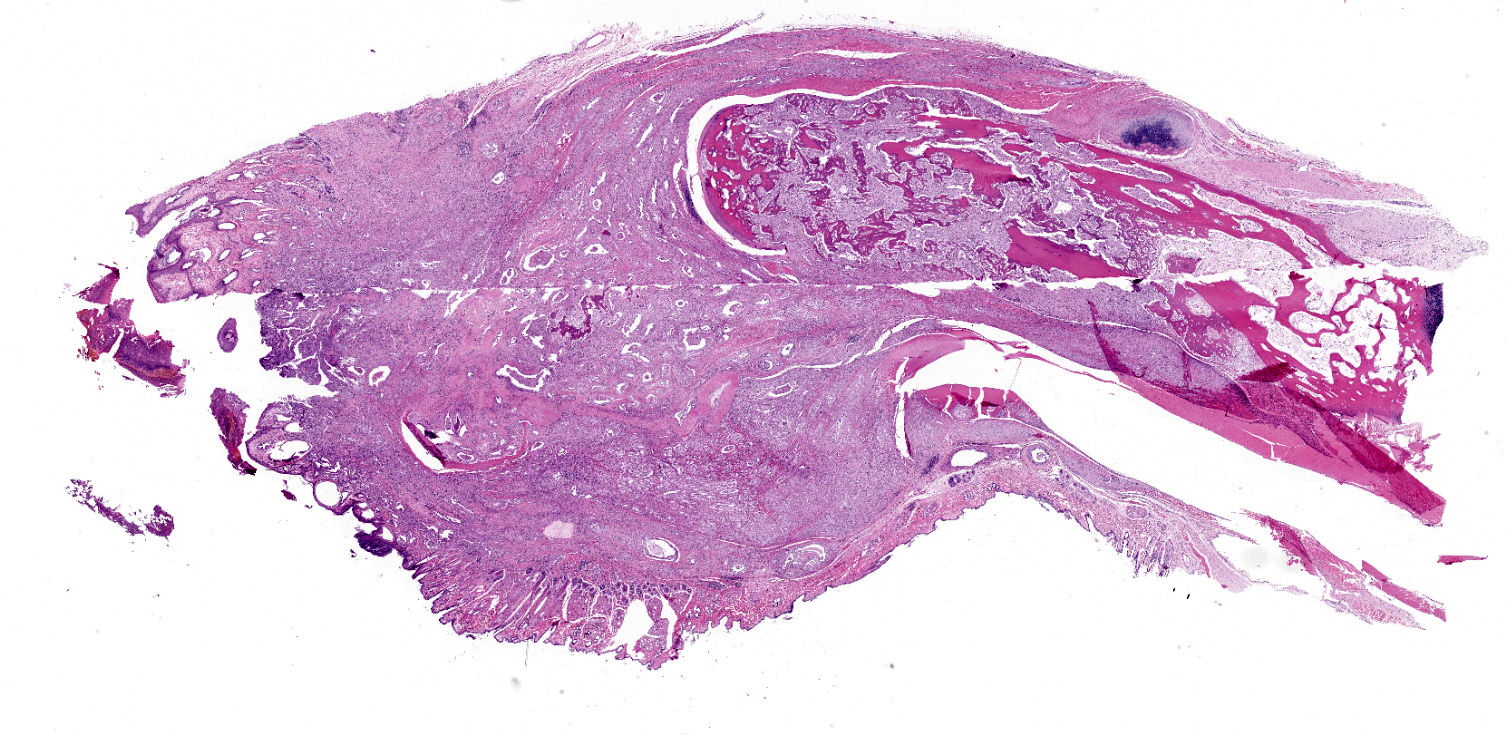
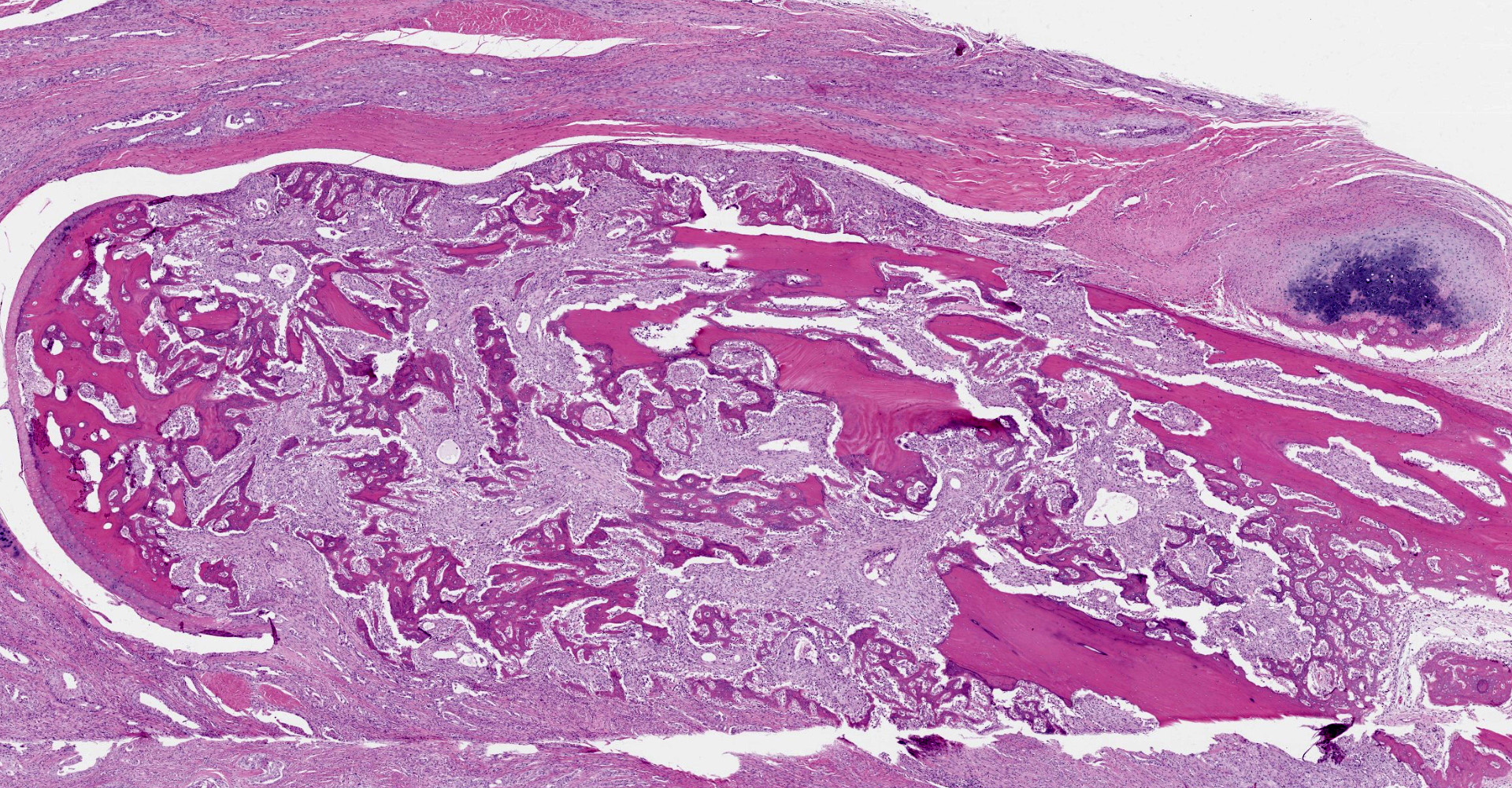
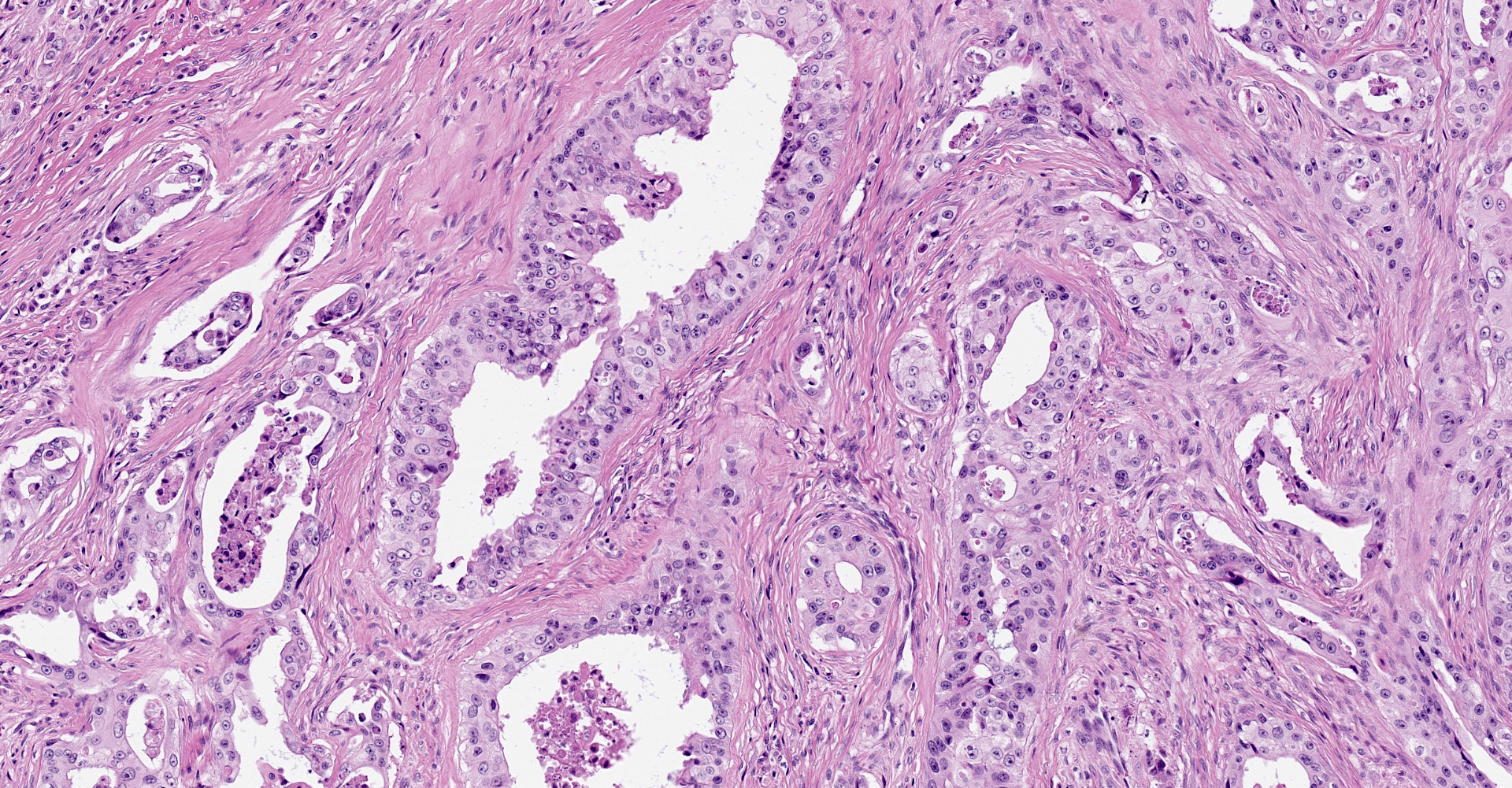
Distal phalanx: Decalcified sections of the distal phalanx are characterized by marked expansion and effacement of the soft tissue and extensive infiltration and of periosteum and bone by a poorly demarcated, moderately cellular neoplasm comprised of respiratory epithelial cells arranged in variably ectatic glandular structures supported and subdivided by a dense desmoplastic stroma. Neoplastic cells are columnar to cuboidal with variably discernible cilia that line the apical surface. Cells have distinct cell borders, moderate amounts of eosinophilic cytoplasm, and round to oval, basilar nuclei with finely stippled chromatin and one prominent nucleolus. There is marked anisocytosis and anisokaryosis. The mitotic count is 13 per 10 high powered fields, 400x. There are many large, discrete to coalescing cystic structures containing central lakes of eosinophilic secretory material admixed with pyknotic to karyorrhectic debris and lined by a single layer of neoplastic respiratory epithelium. Some cystic structures contain aggregates of degenerate neutrophils. The neoplasm effaces the periosteum and cortical bone and infiltrates the medullary cavity. Spicules of partially necrotic bone are frequently lined by the previously described neoplastic cells and/or less frequently lined by few multinucleated osteoclasts. Within the desmoplastic stroma of the neoplasm, there are moderate numbers of neutrophils, macrophages, and occasional loose aggregates of lymphocytes and plasma cells. The neoplasm markedly expands the subcutis and deep dermis and extends to the superficial dermis multifocally.
Contributor's morphologic diagnosis:
Bone, distal phalanx: Metastatic bronchogenic adenocarcinoma.
Contributor's comment:
Feline lung-digit syndrome (FLDS) was originally described by Moore and Middleton from a series of three cases in 1982.9 The main feature was postmortem detection of a primary pulmonary adenocarcinoma, in the face of non-respiratory signs (e.g., dyspnea, coughing, etc). Two presented with pain and swelling of distal extremities and eventually developed respiratory signs in the following weeks. The third case had non-specific clinical findings (hyporexia and weight loss) and was euthanized the following week due to deteriorating quality of life. The two cases with soft tissue swelling were diagnosed as metastatic adenocarcinoma following antemortem biopsy. A retrospective study of 36 additional cats coined the moniker FLDS in 2000.7 All cats presented with metastatic digital carcinoma; again, none had respiratory signs.
While FLDS is classically acknowledged clinically by metastases to extremities, variations exist. Digits are the most common site, but metastases have been documented in skeletal muscle, bone, eye, and skin.5,12 The predilection for digit metastasis may be related to the angioinvasive behavior of the neoplasm, and the highly vascular nature of feline digits, which helps to dissipate heat.5,9 Tumor emboli may mimic aortic thromboembolism (ATE) that is usually attributed to primary cardiac disease. Retrospective studies of 127 cases of ATE identified neoplasia as the cause of 6% of ATE, representing the second most common cause in cats.8 Ischemia and necrosis of the distal limbs may also manifest as a result of thromboemboli.11 Ultimately, the size of thromboemboli dictates where they might settle and the pathophysiology.
Diagnosis is most easily accomplished through biopsy or fine needle aspirate of superficial masses and thoracic radiographs depending on the size of the tumor. The primary tumor often escapes detection due to absence of respiratory signs. More modern imaging technique such as computed tomography may be more sensitive in detecting the primary tumor.12 FLDS is difficult to treat; chemotherapy is not often pursued, as efficacy has not been well documented.12 Amputation of affected digits or limbs, while performed, is rarely palliative.7,10 Increased CK may be useful as a marker of metastases to skeletal muscle. Prognosis is poor to grave, even with pulmonary lobectomy. Mean survival time from initial presentation is 34-58 days.7,13 No breed or sex predilection has been identified.7
In general, primary pulmonary tumors are rare in cats. Of these, adenocarcinoma is most common. Eighty-eight percent of carcinomas in digits are the result of metastases from primary pulmonary carcinomas, although squamous cell carcinomas contribute to a fraction of these cases.13 Lameness is often a common presenting complaint, and lysis of the third phalanx should provide a high index of suspicion;12 however, other differentials should be considered including bacterial and fungal osteomyelitis. One in eight feline nail and nail bed disorders are neoplastic.5 Given that other etiologies have better prognoses and generally response to amputation (i.e., bacterial/fungal osteomyelitis) or have a higher mean survival time (i.e., squamous cell carcinoma ? 207 days), it is advisable to diagnose or rule out primary pulmonary carcinoma as a cause of clinical signs before pursuing surgery.7,13
Contributing Institution:
University of Wyoming/Wyoming State Veterinary Laboratory
JPC diagnosis:
Digit: Metastatic pulmonary carcinoma.
JPC comment:
When feline lung digit syndrome (FLDS) occurs, one of the most common sites of metastasis is to the dermis on the dorsum of the distal phalanx and under the footpad epidermis.2
An important differential to consider is eccrine gland carcinoma.6 Eccrine adenomas and adenocarcinomas have been documents in dogs, while eccrine tumors are usually malignant in cats.4 While a common disease in humans, eccrine glands are confined to the footpads of dogs and cats, and this is the only site at which eccrine neoplasia occurs. Importantly, eccrine and pulmonary tumors have different immunohistochemical profiles. Thyroid transcription factor 1 (TTF-1) is expressed by primary pulmonary carcinoma/adenocarcinoma, in addition to napsin A and keratin 7.3 Approximately 50% of eccrine carcinomas express S100, and about 25% of eccrine and apocrine tumors cells express p63 and has not been documented in metastatic adenocarcinomas in the skin.3
Human primary tumors often metastasize but are likely under reported. Approximately 20-70% of patients who died of their malignant disease had histologic evidence of osseous metastasis at autopsy, but only 2% have metastatic lesions to the foot. These lesions are reported to be CK7 and CDX2 (caudal type homeobox transcription factor 2) immunopositive, consistent with bronchogenic adenocarcinoma.1
The moderator led a discussion about the most common differentials for digital lesions in dogs and cats. In dogs, the most common include subungual melanoma, subungual squamous cell carcinoma, subungual keratoacanthoma, eccrine carcinoma, soft tissue sarcoma, mast cell tumor, trauma/infection, and lupoid onychodystrophy.14 In the cat, the most common digital lesions include lung-digit syndrome, squamous cell carcinoma, various sarcomas, plasma cell pododermatitis, and arteriovenous fistula.15
References:
1. Bazrafshan S, Pacheco M, Ortiz JC. Underlying adenocarcinoma of the lung metastasizing to the proximal phalanx of the food causing complex regional pain syndrome. Journal of the American Podiatric Medical Association. 2017;107(2):150-154.
2. Caswell JL, Williams KJ: Respiratory system. In: Maxie MG ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2, 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:495-497.
3. Dabbs D. Immunohistology of Skin Tumors. In: Diagnostic Immunohistochemistry Theranostic and Genomic Applications. 4th ed. Philadelphia, PA: Elsevier Inc. 4th ed. 2014:414-419, 483-484.
4. Fuentealba C, Illanes O, Haines D. Eccrine adenocarcinoma of the footpads in 2 cats. Can Vet Jour. 2000:41:401-403.
5. Goldfinch, N, Argyle, DJ. Feline lung-digit syndrome: unusual metastatic patterns of primary lung tumours in cats. J Feline Med Surg. 2012;14(3):202-208.
6. Goldschmidt MH, Goldschmidt KH. Epithelial and Melanocytic Tumors of the Skin. In: Meuten DJ, ed. Tumors in Domestic Animals, 5th Ed. Ames, IA:John Wiley and Sons, Inc. 2017;123.
7. Gottfried, SD, Popovitch, CA, Goldschmidt, MH, Schelling, C. Metastatic digital carcinoma in the cat: a retrospective study of 36 cats (1992-1998). J Am Anim Hosp Assoc. 2000;36(6):501-509.
8. Hogan, DF, Dhaliwal, RS, Sisson, DD, Kitchell, BE. Paraneoplastic thrombocytosis-induced systemic thromboembolism in a cat. Journal of the American Animal Hospital Association. 1999;35(6):483-486.
9. Moore, AS, Middleton, DJ. Pulmonary adenocarcinoma in three cats with nonrespiratory signs only. J Small Anim Pract. 1982;23:501-509.
10. Salgüero, R, Langley-Hobbs, S, Warland, J, Brearley, M. Metastatic carcinoma in the ulna of a cat secondary to a suspected pulmonary tumour. J Feline Med Surg. 2012:14(6):432-435.
11. Sykes, JE. Ischemic neuromyopathy due to peripheral arterial embolization of an adenocarcinoma in a cat. J Feline Med Surg. 2003;5(6):353-356.
12. Thrift, E, Greenwell, C, Turner, A-L, Harvey, AM, Maher, D, Malik, R. Metastatic pulmonary carcinomas in cats ('feline lung-digit syndrome'): further variations on a theme. JFMS Open Rep. 2017;3(1):1-8.
13. van der Linde-Sipman, JS, van den Ingh, TS. Primary and metastatic carcinomas in the digits of cats. Vet Q. 2000;22(3):141-145.
14. Wobeser BK, et al. Diagnoses and clinical outcomes associated with surgically amputated canine digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol. 2007;44(3):355-361.
15. Wobeser BK, et al. Diagnoses and clinical outcomes associated with surgically amputated feline digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol. 2007;44(3):362-365.