Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 15
22 January 2020
Dr. Cory
Brayton, DVM, PhD, DACVP, DACLAM,
Director, Phenotypic Core
Associate Professor of Molecular and Comparative and Pathobiology
Johns Hopkins School of Medicine
733 N. Broadway
Baltimore MD, 21205
CASE III: 17-1438 1-2 (JPC 4101223)
Signalment: Two-month-old, intact female mouse, Mus musculus.
History: The mouse was purchased from a non-conventional vendor (pet shop) and it was enrolled in a study as model of autoimmune colitis. The animal was submitted for euthanasia and necropsy given the loss of body weight (10% in the previous 2 weeks) and hunched posture. No other mouse in the group, from the same source and in the same experimental conditions, exhibited any similar or different sign of disease.
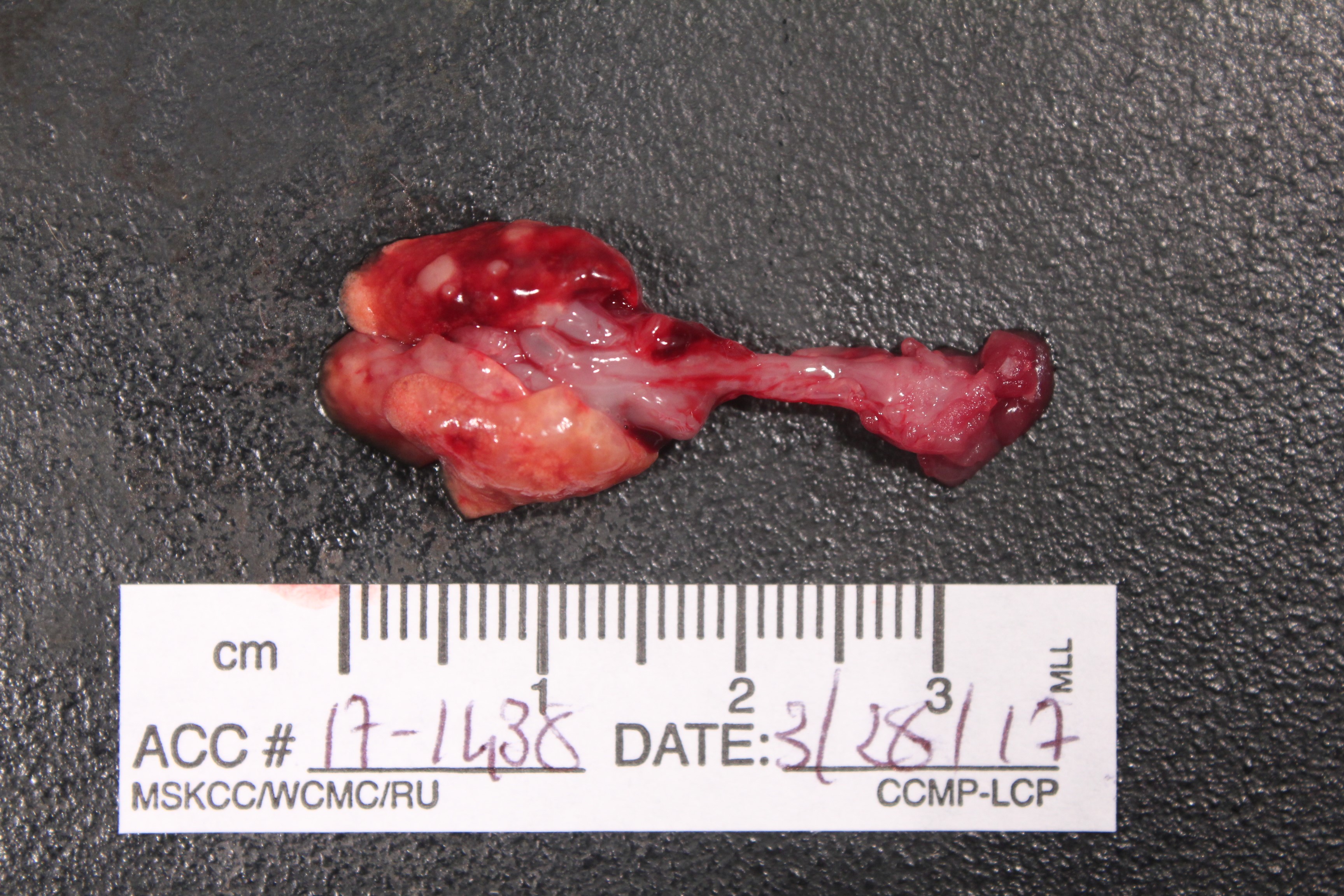
Gross Pathology: The lung lobes are diffusely expanded, firm and mottled (Fig.1). Within the cranial regions there are multiple grey-white nodules measuring about 0.5 to 1 mm in diameter (consistent with bronchiectasis).
Laboratory results: PCR for Mycoplasma spp. on fresh frozen lung tissue was positive
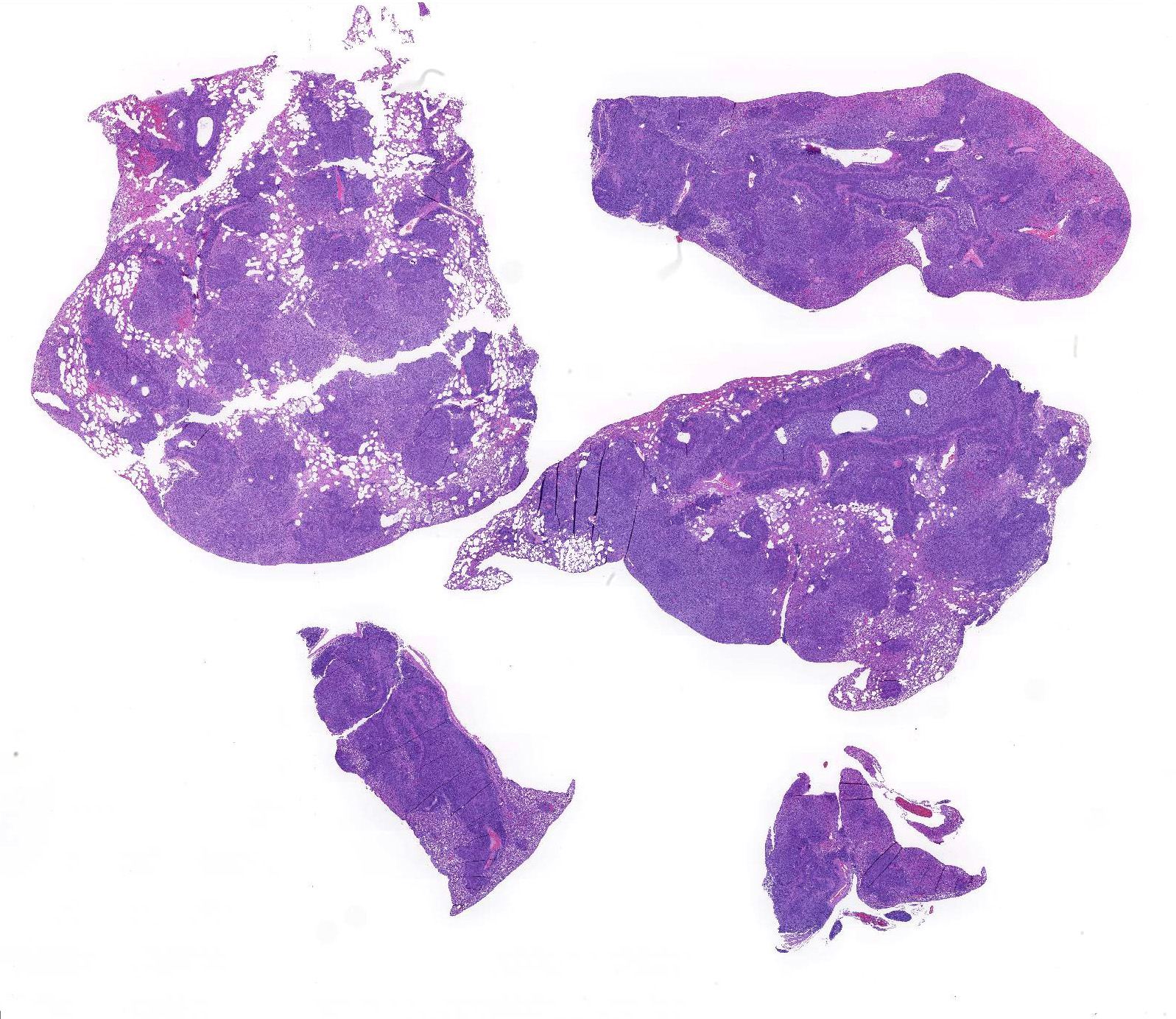
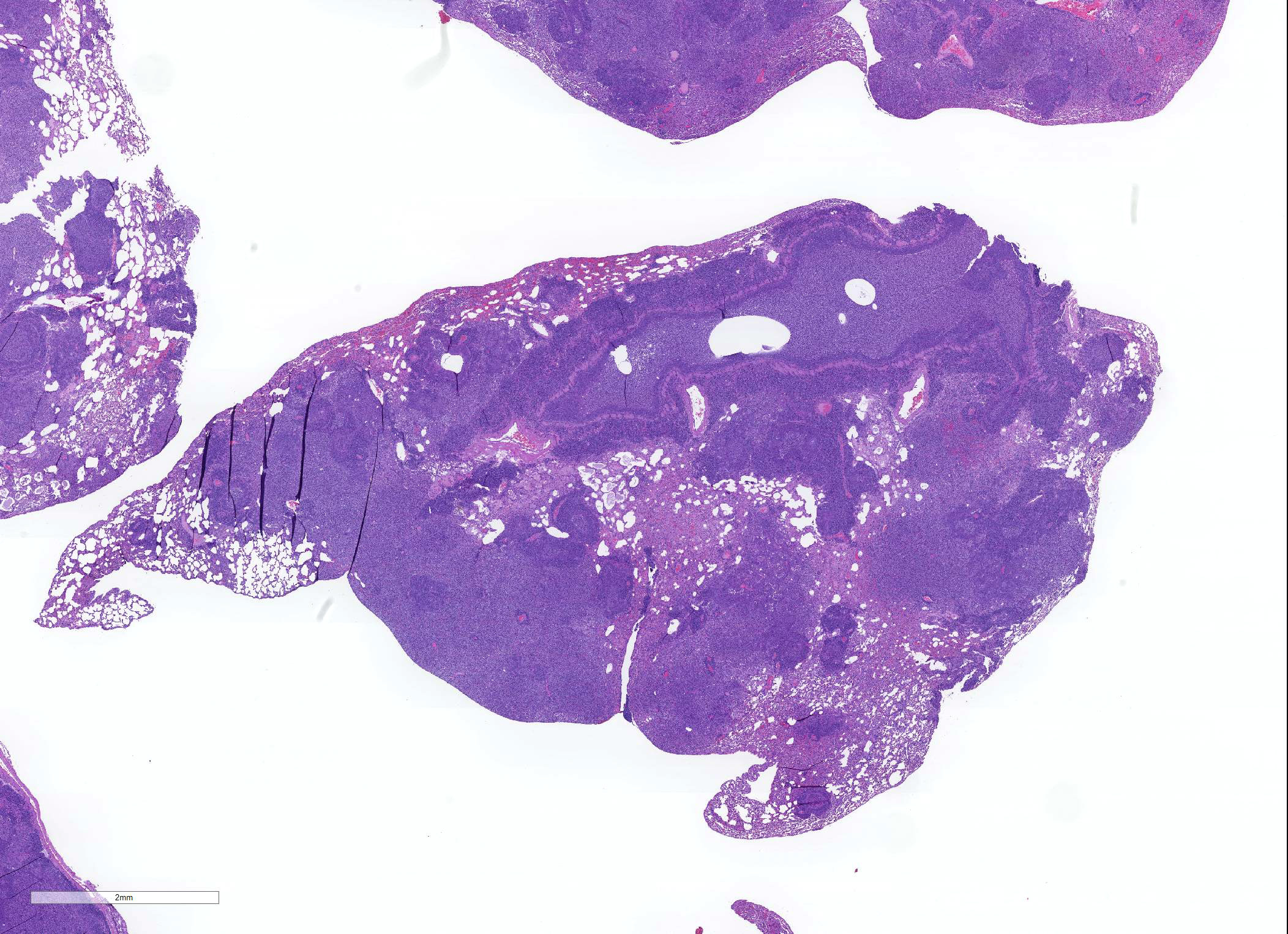
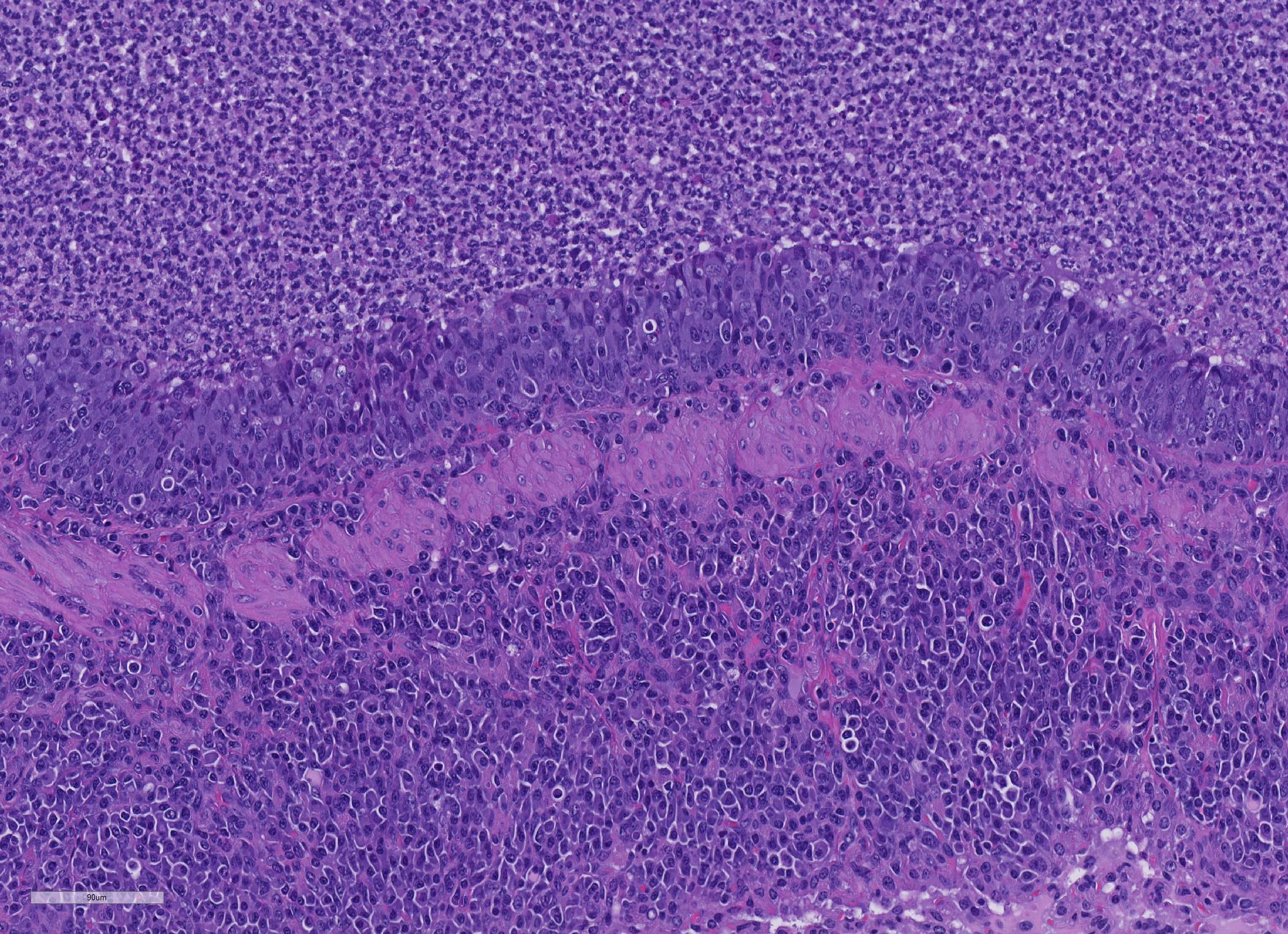
Microscopic Description: About 80 % of the parenchyma is affected in a multifocal to coalescing fashion by the infiltration and accumulation of a large number of mostly viable and occasionally degenerated neutrophils, admixed with few foamy macrophages, obliterating the lumen of alveoli, bronchioles and bronchi. The adjacent parenchyma is atelectatic. Few bronchial and bronchiolar structures exhibit distorted outlines with dilation (bronchiectasis and bronchiolectasis), thickening of the lining epithelium with piling up of nuclei (hyperplasia), and luminal narrowing by polypoid-like structures, formed by a fibrous stalk and lined by one or more layer of cuboidal to columnar epithelium (bronchiolitis obliterans). Multifocally the respiratory epithelium is replaced by one or more layers of cells with features of squamous epithelium (squamous metaplasia). Large cuffs composed of lymphocytes and plasmacells surround the intrapulmonary branches of the bronchial tree. Protein rich fluid is multifocally present within few alveoli (alveolar edema).
Contributor Morphologic Diagnosis:
Lungs: suppurative bronchopneumonia, subacute, severe with bronchiectasis, bronchiolectasis, bronchiolitis obliterans and squamous metaplasia, consistent with M. pulmonis infection
Contributor Comment: Mycoplasma is a genus of bacteria belonging to the order Mycoplasmatales, family Mycoplasmataceae, class Mollicutes. The class name indicates the lack of a wall around the cell membrane, which is a peculiar feature of these bacteria. As such they are classified as gram-indeterminate. Mycoplasmae are divided into 2 clusters: hemotropic and pneumoniae, based on 16S sequencing.
The "pneumoniae" Mycoplasma are often non-pathogenic and are found in the genital and respiratory tracts. Mycoplasma species that are hosted in laboratory mice are: M. pulmonis, M. arthritidis, M. neurolyticum, M. collis, M. muris, however M. pulmonis only is a significant pathogen.
Factors such as Sendai virus infection, high concentration of ammonia in the environment and co-infection with Pasteurella pneumotropica play an important role in the clinical onset of the disease in subclinically infected mice. The modern husbandry standards of mouse facilities and health monitoring plans in research institutions contributed to decrease the incidence of mycoplasmosis despite the rather high percentage of infected animals.
Overall, mice are less susceptible than laboratory rats to the disease, however susceptibility to the disease varies upon the mouse strain, with the B6 being resistant and the C3H quite sensitive. Moreover, females seem to develop more severe forms. The natural disease in immunocompetent mice is usually characterized by weight loss, respiratory symptoms and head tilt or vestibular signs in case of concomitant otitis. Immunodeficient mice on the other hand develop arthritis.8
Colonization of the respiratory tract is mediated by adhesion through special organelles of Mycoplasma organism to cilia, with consequent ciliostasis and retention of mucus.7
Grossly, mucopurulent exudate can be seen in the tympanic bulla, nasal passages and airways. When the disease is subacute or even chronic consolidation of the lung parenchyma and mottling, especially in the cranioventral regions can be appreciated. Variably sized pearl-white to tan nodules can be observed. On cut section, they reveal a wall and a lumen partially or completely obliterated by exudate that correspond to dilated bronchial structures (bronchiectasis).
Histologically, the main and most consistent features are the luminal collection of viable and degenerated neutrophils in the nasal cavities, tympanic bullae, lower airways and the infiltration of lymphocytes and plasma cells forming cuffs around bronchi and bronchioles. Abscess formation as well as squamous metaplasia of the respiratory epithelium can be observed in chronic cases.
The lymphoplasmacytic infiltrate is reported to be more prominent in rats than in mice.8 The mycoplasmal membrane is decorated with high frequency variation antigens which act as superantigens, stimulating the humoral response in the infected host. Lymphoid hyperplasia around the airways is a consistent feature of the enzootic pneumonia of swine, caused by M. hyopneumoniae.7
Mycoplasma have the ability to establish infection thanks to several bacterial factors that prevent phagocytosis and killing by macrophages, which are the first line defense, and increased adherence to host?s mucosal surfaces.3,6,9 Capsular polysaccharide is an antiphagocytic factor.4 Mycoplasma pulmonis produces a polysaccharide called EPS-I which has roles in cytoadherence, protection from complement, inhibition of biofilm formation.1,2 It acts moreover as second shield defense against macrophage binding and phagocytosis. EPS-I appears to provide maximal defense against host response when present together with a long Vsa protein.8 Vsa are a family of surface lipoproteins produced by mycoplasmas.10,11-13 They vary in phase and size. Size variation in particular reduces binding of the bacteria by macrophages beside inhibiting biofilm formation and protecting from complement action similarly to EPS-I.
Contributing Institution:
Laboratory of Comparative Pathology; Hospital for Special Surgery, Memorial Sloan Kettering Cancer Center, The Rockefeller University, Weill Cornell Medicine.
https://www.mskcc.org/research-areas/programs-centers/comparative-medicine-pathology
JPC Diagnosis: Lungs: Bronchopneumonia, suppurative, chronic, diffuse, severe, with marked bronchiolectasis, florid bronchiolar epithelial hyperplasia, and perivascular and peribronchiolar hyperplasia.
JPC Comment: Mycoplasma, in all their minimalized finery, embody much of what we know about what is actually necessary for the development of cellular life. In 1962, the National Aeronautic Space Administration (NASA) embarked on a continuing search for extraterrestial life, and suggesting that if found, it would be extremely simple, leading a generation of scholars to look on our own planet for what that form of life would resemble.9 In doing so, the early investigations into mycoplasma, the simplest form of life with the ability of independent growth in artificial media, were purued.
Mycoplasa are
indeed the essence of a "stripped down" life form, considered a "minimal"
cell. They did not start as simple organisms at the base of the evolutionary
tree which did not progress over billions of year, but evolved contrary to
typical evolution, shedding large parts of its genome, as well as an
independent lifestyle for a very specialized parasitic one, becoming ever
simpler and representing the absolute minimal requirements for cellular life.
Three species of mycoplasma have the smallest genome of all cellular life
forms, with M. genitalium, a cause of urethritis in humans, having the
smallest genome at 580,076 base pairs (contained in circular DNA).5,9
In order to achieve this amazing housecleaning, mycoplasmas have sacrificed
many of their genes, relegating themselves to a very particular parasitic (or
in the case of some insect mycoplasmas) symbiont lifestyles.
One of the obvious results of the shedding of "excess genes" is their lack of a
cell wall. Unlike most bacteria possess a cell wall, cell membrane, and
cytoplasmic membrane, the evolutionary transition from their presumptive
gram-positive roots, mycoplasma have jettisoned both the cell wall and
cytoplasmic membrane, making themselves osmotically fragile and unable to live
in the extra-organism (or extracellular environment for those species who live
within other cells), but facilitating other interesting behavior such as direct
fusion of their membranes with their host ro target cells to facilitate
homeostatic or cytopathic endeavors).9 (The lack of the a cell wall
is also the reason why mycoplasma are resistant to many traditional
antibiotics, which attack bacterial cell walls.)
Another reduction cementing their parasitic lifestyle is the deletion of all genes involved in amino acid, fatty acid and cofactor biosynthesis. Mycoplasma must receive all nutrients (and in the proper concentrations) from the host to survive, a feautre that prevented their growth in the laboratory for many years. Many continue to be fastidious in their requirements, often failing to grow as a result of mild "overdosing" of required amino acids or other nutrients in media.9 (They are however, excellent parasites of cell cultures, with estimates of up to as many of 80% of cell cultures globally containing living mycoplasmas as "part of doing business".)9
Additional gene
deletions have affected energy metabolism, leaving mycoplasmas to depend on
glycolysis as their main, however ineffective means of energy production. They
also are lacking in genes coding for elements of the Krebs cycle or any
cytochromes, damning these processes as "nice to have" but not essential for
cellular life.9
One other way that mycoplasmas have adopted a stripped down life cycle is their
lack of "redundant" genes. For most cellular functions in traditional
bacteria, such as E.coli, each function as built-in redundancy, with
anywhere from 2-6 separate genes for the same function. Mycoplasmas in
generally possess a single gene for each function, which when knocked out as
part of genomic investigation, most often results in death of the cell, or
rarely, revision to non-pathogenicity.9
With all of this loss of genetic material, it is interesting that M.
genitalium, the cellular organism with the smallest genome in the world,
yet possesses twice the number of genes that investigations in the "minimal
cell set" described by molecular biologists as the requirements for life and
reproduction.5 It would appear that Mycoplasma as a genus
still has some housecleaning to do.
One of the most interesting morphologic changes in this particular slide is the marked hyperplasia of bronchiolar epithelium which appears to extend into adjacent alveoli by lepidic growth. While the cause of this change is not evident, the moderator and other renowned mouse pathologists with whom she consulted on this case suggested the possibility of a concurrent virus which may may not have been tested for, such as Sendai or pneumonia virus of mice.
References:
1. Bolland JR, Dybvig K. Mycoplasma pulmonis Vsa proteins and polysaccharide modulate adherence to pulmonary epithelial cells. FEMS Microbiol. Lett. 2012;331:25?30
2. Bolland JR, Simmons WL, Daubenspeck JM, Dybvig K. Mycoplasma polysaccharide protects against complement. Microbiology. 2012;158:1867?1873
3. Davis JK, Davidson MK, Schoeb TR, Lindsey JR. Decreased intrapulmonary killing of Mycoplasma pulmonis after short-term exposure to NO2 is associated with damaged alveolar macrophages. Amer. Rev. Resp. Dis. 1992;145:406?411
4. Finlay BB, Falkow S. Common themes in microbial pathogenicity. Microbiol. Rev. 1989;53:210?230
5. Glass JI, Merryman C, Wise KS, Hutchison III CA, Sith HO. Minimal cells, real and imagined. Cold Spring Harbor Perspect Biol 2017; 9:a023861.
6. Hickman-Davis J, Michalek SM, Gibbs-Erwin J, Lindsey JR. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-sensitive C3H mice. Infect. Immun. 1997;65:2278?2282
7. Maxie G. Jubb Kennedy Palmer?s Pathology of Domestic Animals 6th ed. Elsevier; 2015 vol. 2: 533-35.
8. Percy DH, Barthold SW. Mouse. In: Percy DH, Barthold SW , eds. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Blackwell; 2007: 3?124.
9. Razin S, Yogev D, Naot Y. Molecular Biology and Pathogenicity of Mycoplasmas. Micorbiol Mo Biol Rev 1998; 1094-1156.
10. Shaw BM, Daubenspeck JM, Simmons WL, Dybvig K. EPS-I polysaccharide protects Mycoplasma pulmonis from phagocytosis. FEMS Microbiol Lett. 2013 Jan;338(2):155-60. doi: 10.1111/1574-6968.12048.
11. Shaw BM, Simmons WL, Dybvig K. The Vsa shield of Mycoplasma pulmonis is antiphagocytic. Infect. Immun. 2012;80:704?709
12. Simmons WL, Dybvig K. The Vsa proteins modulate susceptibility of Mycoplasma pulmonis to complement killing, hemadsorption, and adherence to polystyrene. Infect. Immun. 2003;71:5733?5738.
13. Simmons WL, Denison AM, Dybvig K. Resistance of Mycoplasma pulmonis to complement lysis is dependent on the number of Vsa tandem repeats: shield hypothesis. Infect. Immun. 2004;72:6846?6851.
14. Simmons WL, Dybvig K. Biofilms protect Mycoplasma pulmonis cells from lytic effects of complement and gramicidin. Infect. Immun. 2007;75:3696?3699.