Wednesday Slide Conference, Conference 7, Case 4
Signalment:
1-year-old, spayed female, domestic shorthair cat (Felis catus)
History:
This patient presented to the Cardiology Service for evaluation of a heart murmur. An angiogram diagnosed a large, bidirectional patent ductus arteriosus (PDA). On subsequent recheck echocardiograms, shunting transitioned between left-to-right and bidirectional, as well as showed progressive right ventricular hypertrophy. Abdominal ultrasound showed development of mild ascites. At final presentation, this patient developed marked abdominal effusion and abdominocenteses with fluid analysis revealed a high-protein, exudative effusion. Euthanasia was elected due to progressive vomiting and anorexia.
Gross Pathology:
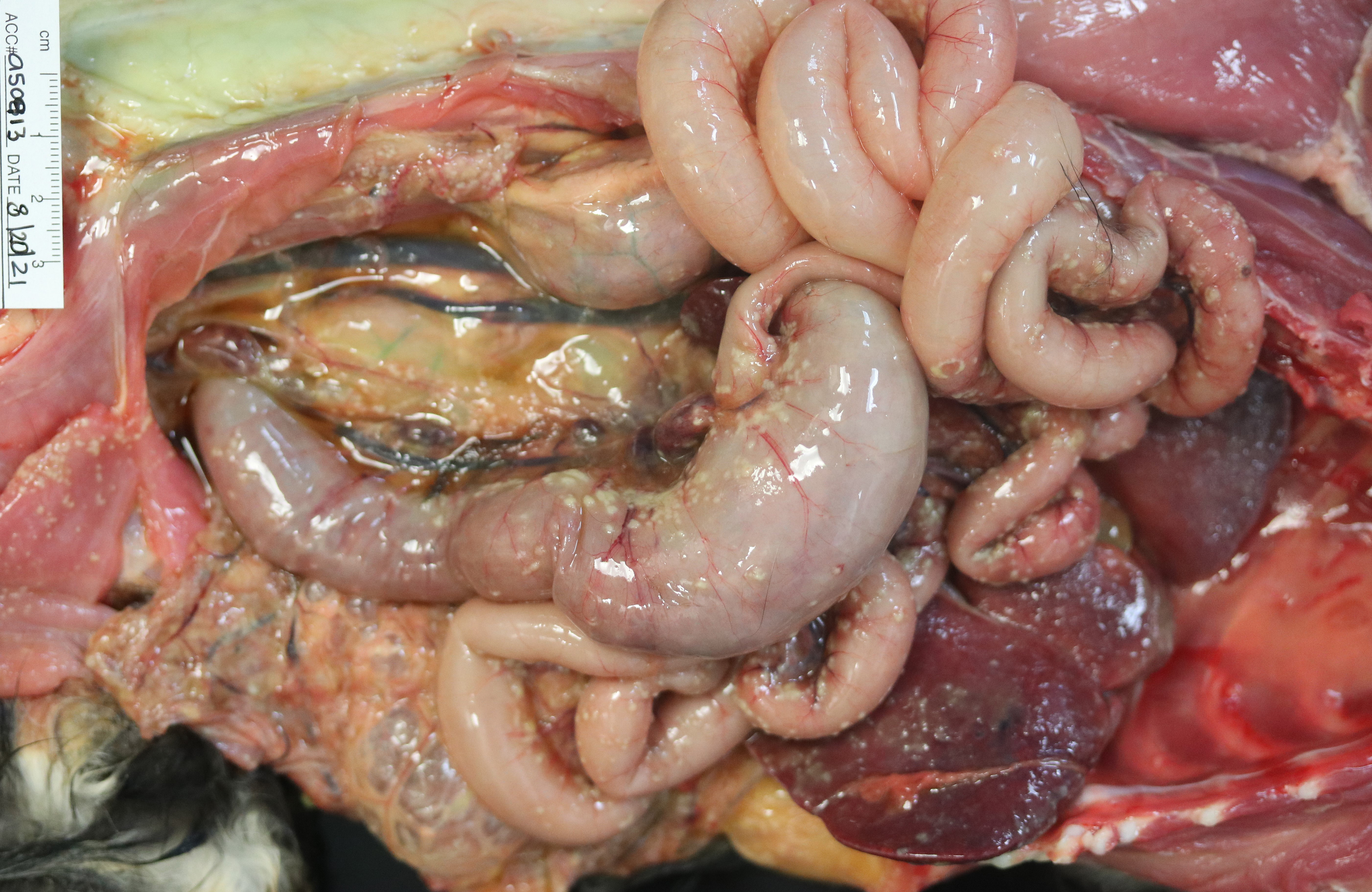
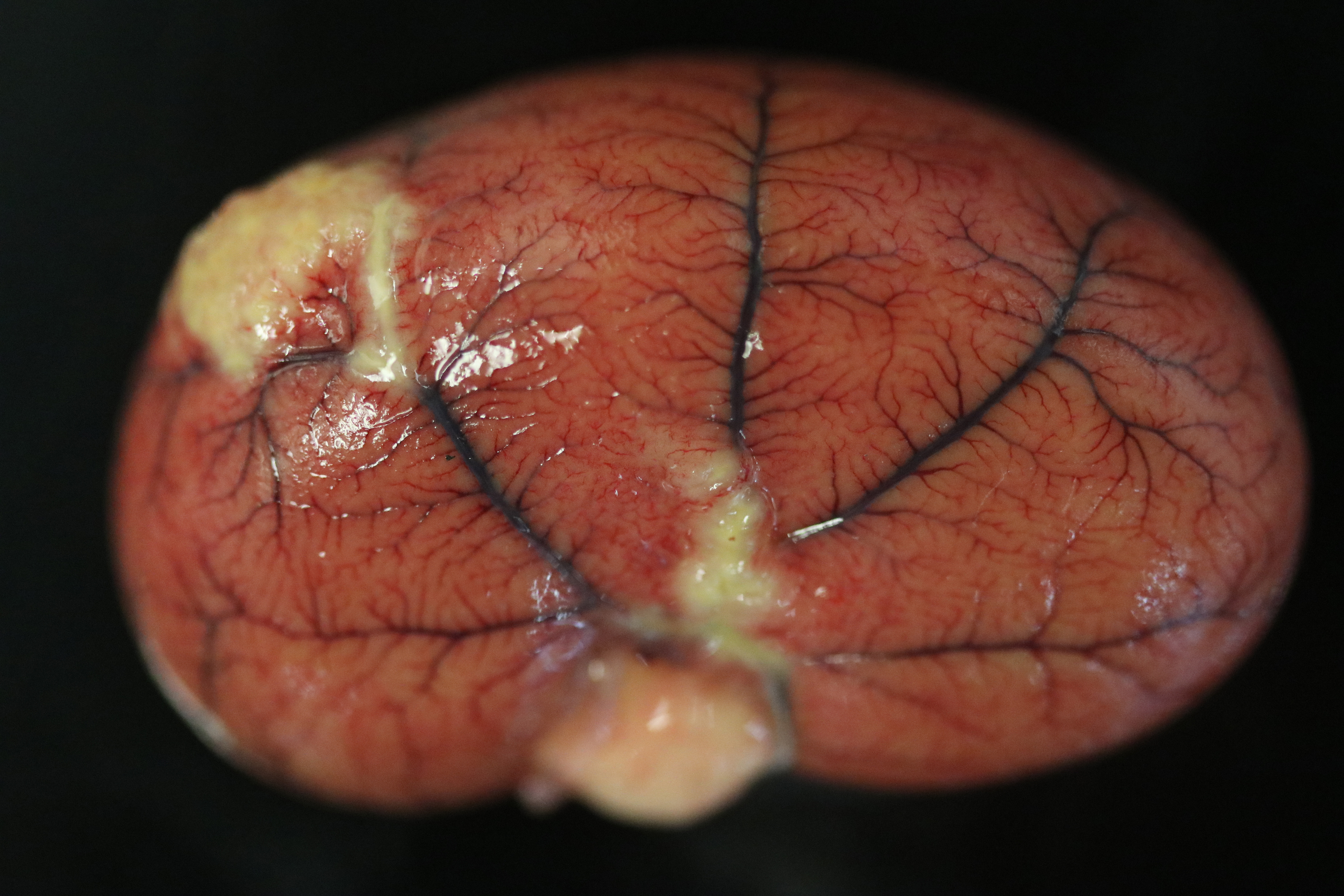
Examination of the lungs is after formalin fixation. The visceral pleura is multifocally expanded by dozens of sharply demarcated, pinpoint to 0.4 cm in diameter, hemispheric, white-tan nodules. On section, the lungs contain dozens of poorly demarcated, firm, tan areas. Lung lobes sections float in formalin.
Extrapulmonary lesions include myriads of multifocal to coalescing, hemispheric, tan-yellow nodules over the peritoneum, omentum, serosa, splenic capsule, hepatic surface, renal capsules, and renal cortices, which range from pinpoint to 1 cm x 0.6 cm x 0.6 cm. Nodules often track along mesenteric and renal subcapsular veins. The mesenteric, hepatic, pancreaticoduodenal, and colonic lymph nodes are moderately to severely enlarged and the cut surfaces contain dozens of coalescing, poorly demarcated, tan-yellow regions. Within the abdominal cavity is approximately 80 mL of a viscous, mildly turbid, yellow fluid mixed with dozens of gelatinous, semi-translucent, yellow strands that are loosely adhered to the viscera (fibrin). The serosae are rough and dull and hepatic and splenic capsules are multifocally cloudy and dull.
Laboratory Results:
Albumin: 1.9 g/dL (reference 2.6 - 3.9 g/dL)
Globulin: 6.2 g/dL (reference 3.0 - 5.9 g/dL)
Albumin: Globulin Ratio: 0.3
Abdominal effusion: Fluid analysis: high-protein (greater than 4.0 g/dL), exudative effusion
Abdominal Fluid - Feline Coronavirus RealPCR: Positive (Biotype FECV)
Microscopic Description:
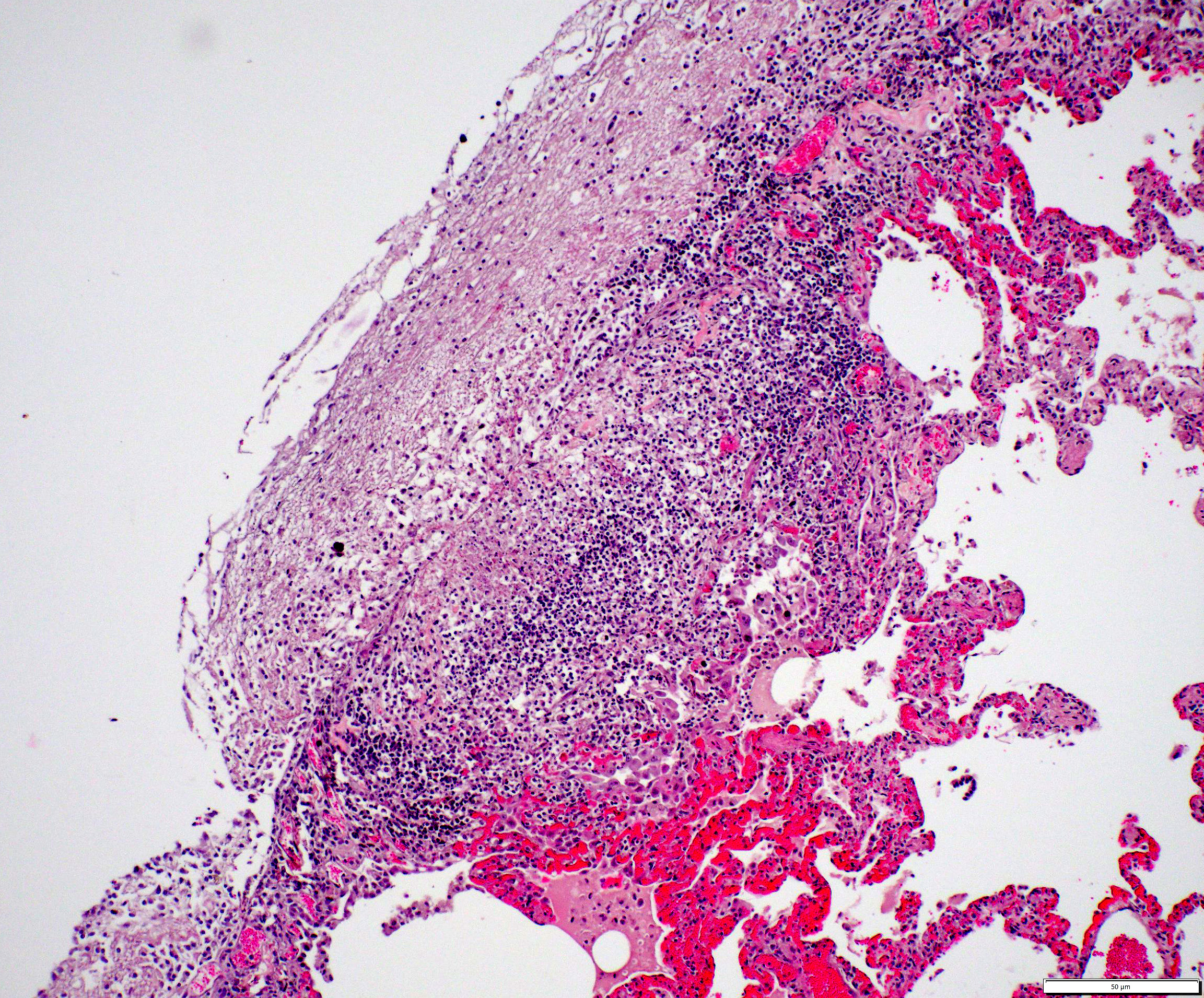
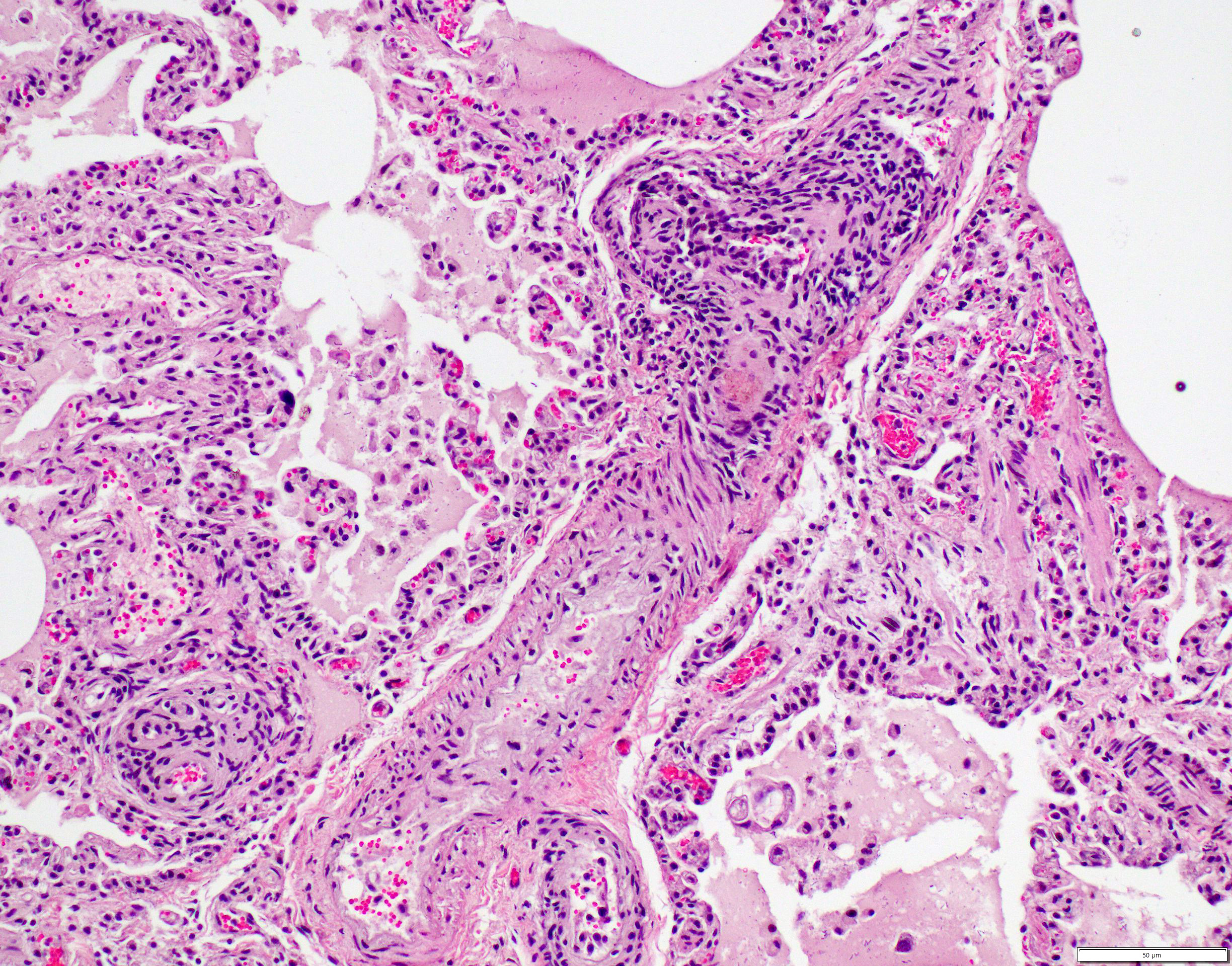
Lungs: The submitted sections of lung show multiple overlapping pathologic processes. The pleura is multifocally elevated and expanded by an acellular, fibrillar, eosinophilic material (fibrin) mixed with karyorrhectic debris (necrosis) and moderate to large numbers of lymphocytes, plasma cells, macrophages, and neutrophils. Inflammation infiltrates and expands the subjacent alveolar septa, which are frequently lined by hyperplastic type II pneumocytes. Expanding and infiltrating the remaining alveolar septa are moderate numbers of macrophages, lymphocytes, plasma cells, and neutrophils with rare type II pneumocyte hyperplasia. Filling the alveolar spaces is variable pale eosinophilic (seroproteinaceous) fluid, extracellular bacteria (mixed morphologies), increased intra-alveolar macrophages, and intra-alveolar protein.
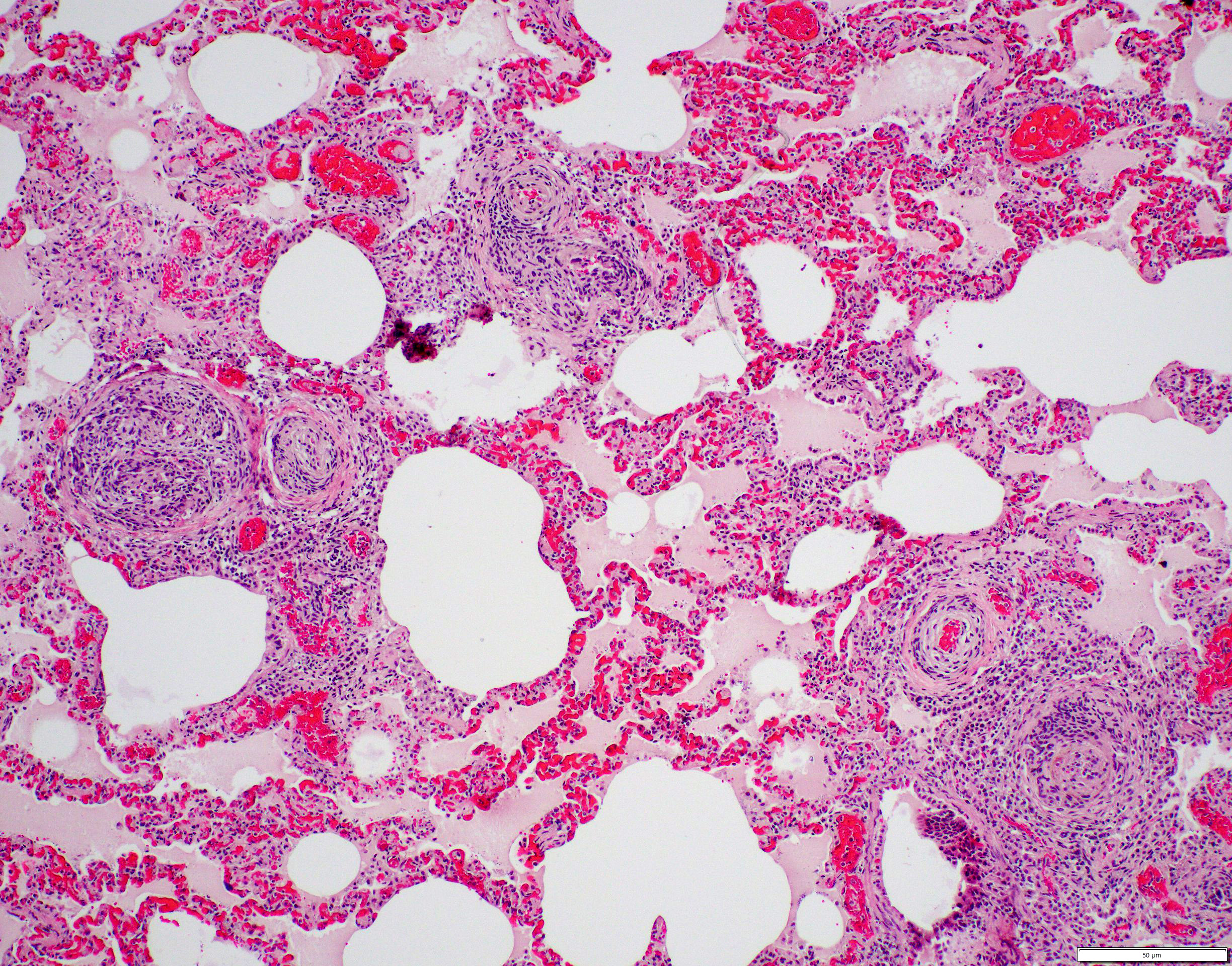
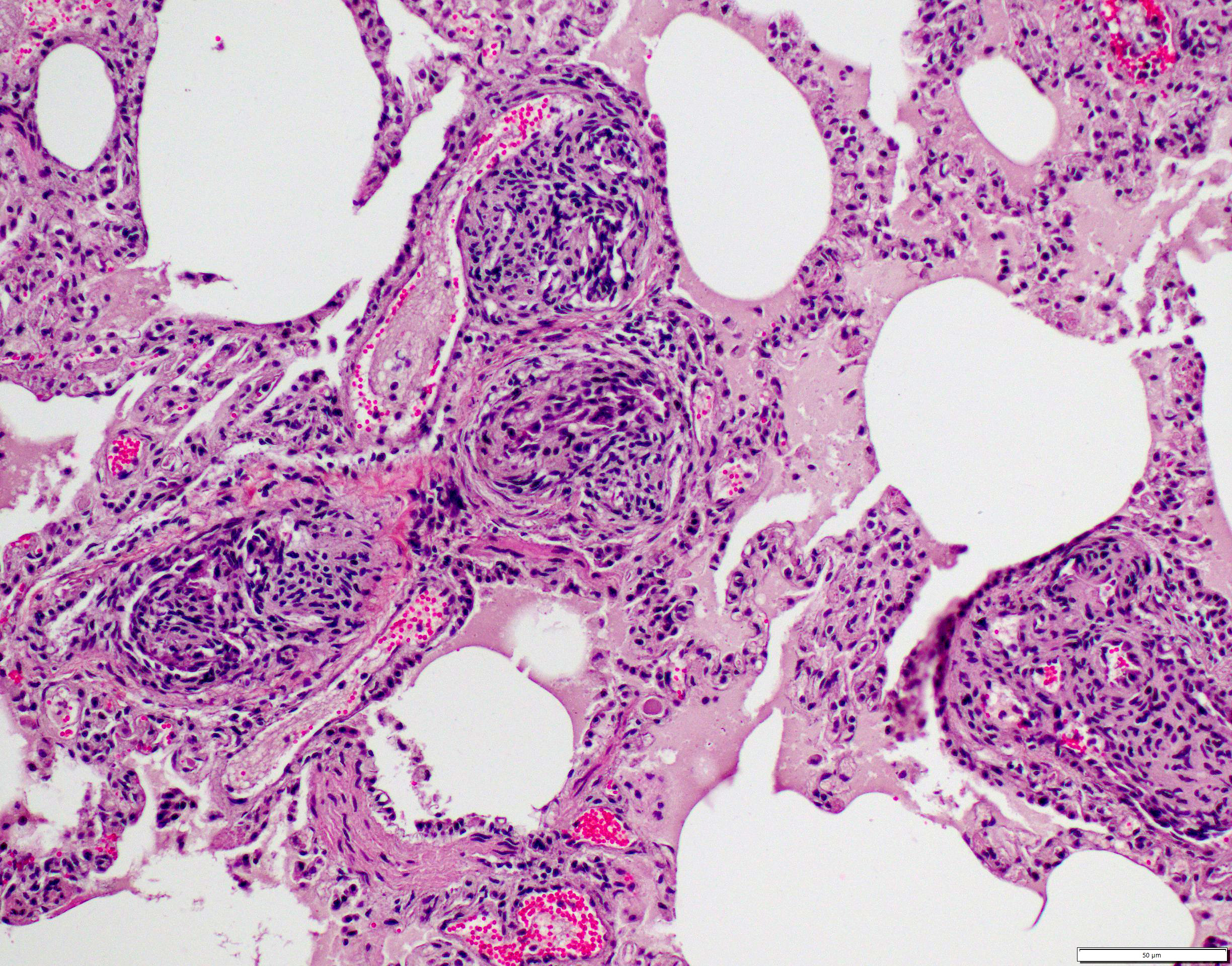
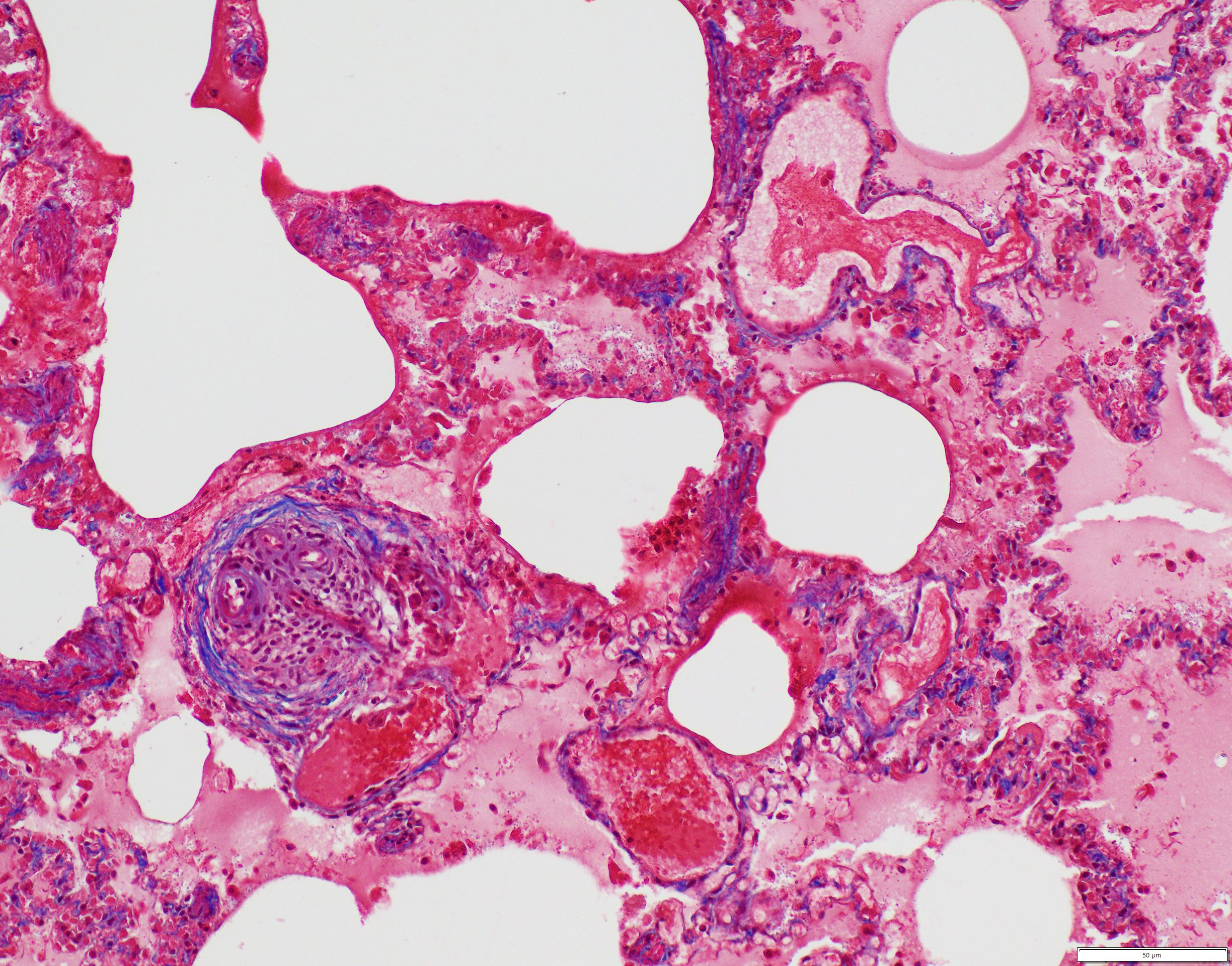
Small to medium-sized pulmonary arteries/arterioles display multiple pathologic changes. The tunica intima and subintima are partially to circumferentially expanded by a homogenous, eosinophilic matrix (fibrosis). The tunica media is multifocally expanded by fibrosis and/or increased numbers of hypertrophied smooth muscles cells (muscular hypertrophy) that cause compression/obliteration of the vascular lumens. Vascular lumens are commonly bridged and filled by plexiform lesions characterized by sieve-like masses comprised of a core of an extracellular matrix, smooth muscle cells, and stromal cells lined by quiescent and hypertrophied endothelial cells. When longitudinally sectioned, plexiform lesions are prominent at branch points. Variable expansion of the tunica adventitia is by either concentric dense or loose onion-skin fibrosis. Multiple blood vessels are moderately to severely distended by sac-like dilations (highlighted by Masson’s Trichrome stain). Intermittent blood vessels are cuffed by mild to moderate numbers of lymphocytes, plasma cells, macrophages, and neutrophils. Separating the perivascular adventitia and tissues are clear spaces and an acellular, pale eosinophilic fluid (edema). There is multifocal, mild to moderate vascular congestion. Occasional macrophages contain morphologically separate intrahistiocytic pigments and foreign material (carbon, silica, hemosiderin, and/or lipofuscin).
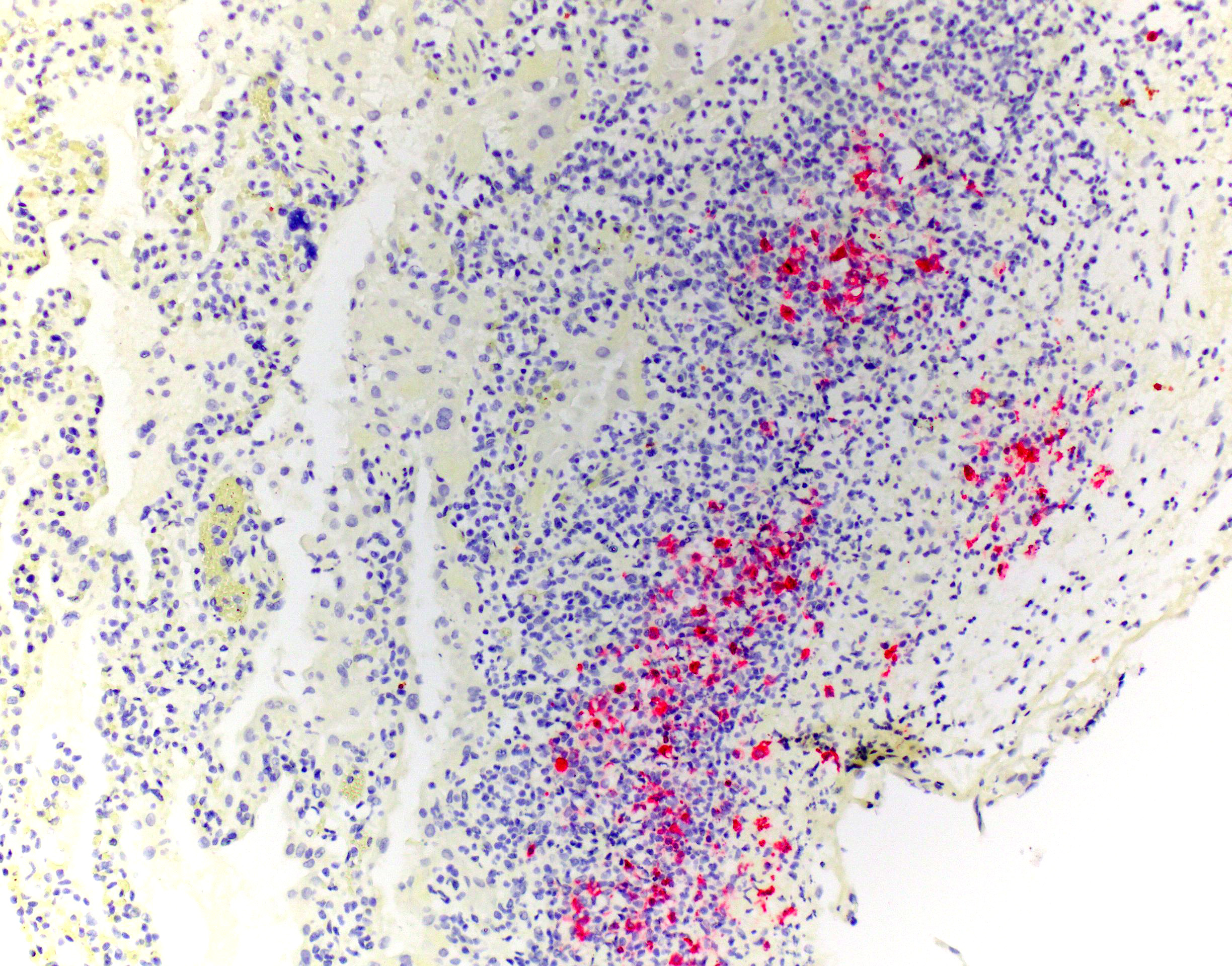
Immunohistochemical stain for FIPV (Animal Health Diagnostic Center/Cornell University) revealed strong cytoplasmic immunoreactivity of macrophages within pleural granulomas.
Contributor’s Morphologic Diagnosis:
Lungs:
- Pulmonary arteriopathy, chronic, multifocal, severe with multifocal, mild to moderate, intimal, subintimal, medial, and adventitial fibrosis; moderate to marked medial hypertrophy; multifocal plexiform lesions; and multifocal, moderate to marked vascular dilation
- Pleuritis and interstitial pneumonia, fibrinonecrotizing, histiocytic, lymphoplasmacytic, neutrophilic, chronic, multifocal to coalescing, moderate with regional type II pneumocyte hyperplasia
- Interstitial pneumonia, histiocytic, lymphoplasmacytic, neutrophilic, chronic, diffuse, moderate with multifocal type II pneumocyte hyperplasia, alveolar histiocytosis, and minimal intra-alveolar protein
- Edema, diffuse, mild to moderate
Contributor’s Comment:
Microscopic examination in this case revealed two distinct pathologic processes consistent with concurrent feline infectious peritonitis virus (FIPV) infection and plexogenic pulmonary arteriopathy. Additional findings consistent with FIPV infection included high-protein abdominal effusion, multiorgan and body cavity inflammatory nodules, fibrinonecrotizing phlebitis, and pleocellular lymphadenitis. Immunohistochemistry confirmed FIPV within lesions. In this patient, systemic-to-pulmonary shunting through the PDA resulted in the prominent plexiform arteriopathy.
Coronaviruses infect a wide-range of wild and domestic species, where they are associated with respiratory, reproductive, gastrointestinal, and systemic diseases.1,7,8,11-14,17 Virions are enveloped and contain large (80-220 nm), positive-sense, single-stranded RNA genome.7,8,11,14,17 Viral proteins include 3-4 structural proteins (spike glycoprotein (S), transmembrane glycoproteins (M and E), nucleoprotein (N), and inconstant hemagglutinin (HE)) and non-structural accessory proteins.7,8,11,17 Club-shaped spike glycoproteins (S) form a characteristic radiating crown-appearance on ultrastructure. 7,8,11 These spike proteins mediate receptor binding and fusion at the plasma membrane or endosomes facilitating viral entry.7,8,11,14 Thus, the host range and cell tropism is predominantly determined by the spike (S) protein.7,8,11,14 In addition, spike proteins participate in induction antibody and cell-mediated responses.8 Coronaviruses exhibit a high mutation rate due to RNA-polymerase transcription errors and genetic recombination between related coronavirus during coinfections.8,11,13,14 Feline coronaviruses (FCoVs) belongs to the family Coronaviridae within the alphacoronavirus genus.7,8,11,17 Other important alphacoronaviruses in veterinary medicine include canine coronavirus, TGE virus of swine, porcine epidemic diarrhea virus (PEDV), porcine respiratory coronavirus, alpaca respiratory coronavirus, and ferret and mink coronaviruses.7,11 There are two main circulating serotypes of feline coronaviruses (FCoVs): the predominant serotype I and rare serotype II.7,8,11,13,14,17 Infection with serotype II is initiated through binding of the host aminopeptidase N receptor but the receptor for serotype I remains unknown.7,8,11,13,17 A potential other host receptor, dendritic cell-specific ICAM (DC-ICAM), has been suggested for both serotypes.7,8,13
Feline coronaviruses (FCoV) include two separate biotypes: feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV).1,7,8,11,12 FECV infects and replicates in the intestinal epithelium and is generally associated with a self-limiting, mild gastroenteritis.1,8,11-13 A severe catarrhal and hemorrhagic enteritis can be seen in juveniles.7,8 The main mode of transmission for FECV is fecal-oral but transmission can occur via direct contact (saliva, grooming behavior), respiratory droplets, and maternal shedding.1,8,11,17,18 Meanwhile, FIPV infects and replicates in monocytes/macrophages and is associated with a severe, multisystemic immunoinflammatory disease.1,7,8,11,13 The amount of replicating virus shed in FIPV-positive cats is extremely low with diminished replication in the enterocytes, therefore capacity for horizontal transmission is unlikely.8,14 Feline infectious peritonitis (FIP) is an invariably fatal disease of both domestic and wild felids.1,6-8,11-13,15,16 Outbreaks are related to environmental (crowding, concurrent infections, long-term exposure to shedders), virus, and host (individual immune response) factors.8,13The prevailing thought is that FIPV results from a mutated feline enteric coronavirus (in vivo mutation transition/internal mutation theory). 1,6-8,10-18 A single mutation has not been identified, rather multiple mutations associated with the 3C accessory gene and fusion and binding domains of the spike (S) protein are common in virulent biotypes.1,7,8,11,13,14,17 Significant mutations in the FECV genome facilitates switching to FIPV by imparting tropism for macrophages, as well as productive and sustained replication in macrophages.1,6-8,10-18 Acquired virulence factors allow for dissemination via leukocyte trafficking.1,7,8,12-14,17 An alternative, less prominent theory states that FIP infections are due to distinct circulating avirulent and virulent strains, which may be the case in rare outbreaks.8,14,16 FIP typically occurs in young or geriatric cats, with multi-cat environments, stress, and immunosuppression known risk factors.7, 8,11,12,16 Increased incidence is reported in Abyssinians, Bengals, Birmans, Himalayans, Ragdolls, and Rex breeds.7,8,11
Individual immune-responses play an essential role in FIPV disease progression.7,8,13,14 Protective immunity is considered cell-mediated.8,14 Severe disease is linked to a strong humeral response that is ineffective in virus elimination and simultaneous weak cell-mediated response.1,7,8,11-14,17 Depletion and reduced cytotoxicity of NK cells coupled with decreased regulatory T-cells (Tregs) are seen in FIP cats.8,14 In FIP infections a robust antibody response increases the severity of the disease, a phenomenon known as antibody dependent enhancement. 7,8,12-14,17,18 Classic FIP manifestations are “wet”/effusive and “dry”/pyogranulomatous forms, but a combination of the two is common.1,7,8,11-14,16 The individual animal’s immune response is thought to direct the spectrum of pathology.1,7,8,11-14,16 With a vigorous cell-mediated response FIP lesions do not develop, while in patients who lack a cell-mediated response and have a strong humoral response the “wet”/effusion form develops.1,8,12-14,17 The “dry”/pyogranulomatous form is associated with an intermediate response. 1,7,8,14,17 Typical gross pathology seen in the “wet” form is a fibrinous polyserositis with a viscous, high-protein, yellow exudative effusion with variable parenchymal involvement.1,7,8,11,12, 14,16,17 With the “dry” form distinct granulomas/pyogranulomas throughout the body cavities and viscera are seen, which classically track along blood vessels and are not accompanied by effusion.1,7,8,11,12,14,16,17 Commonly affected organs include the omentum, serosa, peritoneum, kidney, brain, and eye.7,8,18 With brain involvement, meningeal plaques, intraventricular protein and fibrin-rich exudate, hydrocephalus, and cerebellar herniation can be seen. 7,12 Ocular manifestations include conjunctivitis, high-protein fluid in the anterior and posterior chambers, keratic precipitates, and retinal detachment.7,10 Microscopic changes include characteristic vasculocentric, pyogranulomatous to pleocellular inflammation with overt phlebitis and variable vascular necrosis that preferentially involves small to medium size veins.1,7,8,11,12,14,16,17 A similar progressive multisystemic inflammatory disease has been described in ferrets with coronavirus infection, which has been denoted as ferret systemic coronavirus.6,7,11,12,15, 18
Activation of FIP viral-infected macrophages contribute to the characteristic histopathologic changes, perivascular lesion distribution, and vascular damage with resulting vascular leakage.7,8,11,16,17 Activated tissue macrophages secrete proinflammatory cytokines (TNFα, IL1β, IL6, IFNγ, GM-CSF and G-CSF), matrix enzymes (MMP9), and leukocyte adhesion molecules, as well as upregulation of surface integrins (CD18, CD11a, and CD49d) that lead to leukocyte chemotaxis, induce differentiation and proliferation of additional monocytes and neutrophils, intensify the inflammatory reaction, increase vascular permeability, and exacerbate tissue damage.7, 8,11,13,14,16,17 Compliment activation accelerates chemotaxis and leukocyte adhesion.16,17 Increased VEGF transcription and levels in infected cats further increase vascular permiability.8,14 Both immune-mediated type III and type IV hypersensitivity reactions are hypothesized to play a role in the vascular lesions and perivascular inflammation.7, 14 Antigen-antibody immune complexes and antibody-complement complexes deposit in vessel walls and perivascular tissues where they initiate tissue damage and recruit inflammatory cells.7,8,12-14,16,17 Hyperstimulation of T-cells and macrophages, via type IV delayed hypersensitivity, cause perivascular tissues. 7,12,17
Clinical signs associated with FIPV tend to be vague, non-specific, and depends on varying organ involvement. 1,7,11,12,16 FECV and FIPV cannot be distinguished serologically or morphologically making antemortem diagnosis of FIP challenging and therefore clinical signs and antemortem diagnostics need to be evaluated in concert using a diagnostic algorithm.8,14 Bloodwork changes associated with FIP include lymphopenia, regenerative anemia, hypoproteinemia due to hypergammaglobulinemia, and biochemical changes associated with specific organ involvement.8 FIP effusions typically have a high protein content (> 35g/L) with concurrent low cellularity (< 3500 nucleated cells/ml).8 A serum albumin: globulin (A:G) ratio <0.8 is highly suggestive of FIP infection; meanwhile, an effusion A:G ratio <0.4 has a high positive predictive value for FIP.8 Acute phase proteins, including alpha1 acid glycoprotein (AGP), are elevated with FIP.8 Higher titers (≥1:1600) for FCoV-specific antibodies in the blood and effusion are more indicative of FIP.8 RT-PCR can detect virus in feces, blood, and effusions but cannot differentiate between the two biotypes.8 Immunohistochemistry allows for confirmation of coronavirus antigen within lesions on biopsy or postmortem samples, as in this case.11
The prominent vascular changes in this patient are characteristic for plexogenic pulmonary arteriopathy, a set of morphologic changes associated with pulmonary arterial hypertension (PAH). Causes of pulmonary hypertension include idiopathic (primary) PAH, systemic-to-pulmonary vascular shunts (including patent ductus arteriosus), chronic pulmonary thromboembolic disease, disorders of pulmonary blood vessels (e.g., heartworm disease, pulmonary veno-occlusive disease, pulmonary vasculitis), hypoxic vasoconstriction, chronic interstitial lung disease, or pulmonary venous hypertension due to left-sided heart failure.3 In the submitted case, PAH-associated lesions were associated with a known risk factor or condition (i.e., reported PDA).3,19 Altered vascular tone is believed to be the result of imbalanced vasodilatory (e.g., prostacyclin, NO and cGMP) and vasoconstricting (e.g., endothelin, thromboxane A2, and serotonin) molecules.3 Other implicated vasoactive molecules include TGFβ, BMP-2, FGF, and platelet-derived growth factor.3 PAH results in endothelial injury/degeneration, vasculitis, fibrinoid vascular necrosis, smooth muscle proliferation, and “onion skin” perivascular fibrosis.3,5,19 Thus, the vascular remodeling seen with PAH is considered the result rather than cause of hypertension; however, narrowed vascular lumens can exacerbate pulmonary hypertension.3,5,19 Right ventricular pressure overload secondary to increased vascular resistance leads to right ventricular eccentric hypertrophy and ultimately right-sided congestive heart failure.2,19
PAH-associated histopathology represents a spectrum of both constrictive (medial and intimal remodeling) and complex (plexiform and dilative); these include endothelial hypertrophy, intimal, medial, and adventitial fibrosis, muscularization of small arterioles, vasculitis, thrombosis, plexiform lesions, and adventitial edema.2-4,19 In humans, lesions are separated into grade I (muscular hypertrophy), grade 2 (intimal proliferation), grade 3 (concentric laminar intimal fibrosis), grade 4 (necrotizing vasculitis), grade 5 (plexiform lesions), and grade 6 (dilation and angiomatoid lesions) changes.4 Higher grade lesions are typically associated with higher pulmonary artery pressures, although they do not represent a sequential disease progression and all grades can develop independently or concurrently.4, 19 As in this case, characteristic plexiform lesions are characterized by vascular lumens filled by web-like proliferations formed by a core of smooth muscle cells and/or collagenous stroma lined by endothelial cells, which eventually lead to irreversible obliteration of the arterial lumens.2-5,19 Lesion distribution is to the small muscular arteries and arterioles particularly at the branching points.2-5,19 Dilation and angiomatoid lesions tend to occur at the artery/arteriole distal to the lesion suggesting impaired blood flow in their development.2-5 Secondary pulmonary hemorrhages can be seen.4 Plexiform lesions are dynamic structures involving the cross-talk between quiescent endothelial cells, apoptosis-resistant myofibroblasts, smooth muscle cells, and undifferentiated cells at the lesion’s core.2,5 Jet lesions at mouth of branch vessel and necrotizing arteritis may contribute to lesions.5 Plexiform lesions are thought to be chronic attempts to repair injured vessels and likely represent cellular, recanalized thrombi secondary to vascular damage.3-5
Contributing Institution:
Schwarzman Animal Medical Center
510 E. 62nd Street
New York, NY 10065
http://www.amcny.org/
JPC Diagnosis:
1. Lung: Pneumonia, interstitial, lymphohistiocytic and neutrophilic, chronic, diffuse, moderate, with multifocal fibrinonecrotizing pleuritis.
2. Lung, small arterioles: Plexiform (plexogenic) arteriopathy, chronic, multifocal, severe with marked intimal hyperplasia and medial hypertrophy, fibrinoid necrosis, thrombosis, and recanalization.
JPC Comment:
The final case of this conference is quite complex with a plethora of features to put together to arrive at a final understanding of the case. Conference participants did not have access to the gross photos from this case (figures 4-1 and 4-2) beforehand, though they are highly suggestive of FIP and assist with interpretation of microscopic changes. The interstitial pneumonia and pleuritis are expected microscopic correlates (figures 4-3 and 4-4), though the vascular features are initially puzzling as the pulmonary veins lack overt phlebitis in the sections available while smaller pulmonary arteries have features reflecting hypertension (figures 4-5 and 4-6) which is not associated with FIP. Conference participants struggled to nail down how to best capture this feature as the arterial response to hypertension (plexiform) is not as commonly described perivascular concentric lamellated fibrosis (‘onion skin’). Readers that are interested in exploring the genesis of this lesion in detail would greatly benefit from reviewing the excellent summary by Carman et al.2
Briefly, plexiform-type vascular changes arise from nonspecific medial and adventitial thickening of the pulmonary artery with extension of smooth muscle to small non-muscular arteries.2 Subsequent complex remodeling driven by dysfunctional,5 hyperproliferative, and apoptosis-resistant pulmonary endothelial cells leads to aberrant signaling between smooth muscle cells and fibroblasts with the result being progressive obliteration of the lumen of the vessel by laminar, stalk-like, or complete aneurysm-like projections of endothelial cells overlying a core of smooth muscle and/or collagenous stroma.2 HIF-2α and VEGF signaling likely play a role and models of disease have focused on hypoxia as an inciting event in the development of this lesion though other concurrent factors are likely needed to induced complex lesions.2,5 Interestingly, animals with experimentally treated with inhibition of VEGFR and hypoxic conditions that were later returned to normoxia developed large-scale plexiform pulmonary lesions. The interplay of ineffective endothelial apoptosis, strong impetus for angiogenesis, and aberrant cytokines and growth factors derived from a ‘misguided’ endothelial cell ringleader5 is a solid hypothesis though this phenomenon remains incompletely understood.
References:
- Andre NM, Miller AD, and Whittaker. Feline infectious peritonitis virus-associated rhinitis in a cat. Journal of Feline Medicine and Surgery Open Reports. 2020; 1-6.
- Carman BL, Predescu DN, Machado R, et al. Plexiform Arteriopathy in Rodent Models of Pulmonary Arterial Hypertension. The American Journal of Pathology. 2019; 189(6): 1133-1144.
- Caswell JL and Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 492.
- Churg A and Wright JL. Pulmonary Hypertension. In: Leslie KO and Wick MR, eds. Practical Pulmonary Pathology. 3rd ed. Philadelphia, PA: Elsevier; 2018: 404-407.
- Fishman AP. Changing Concepts of the Pulmonary Plexiform Lesion. Physiol Res. 2000; 49: 485-492.
- Garner MM, Ramsell K, Morera N, et al. Clinicopathologic Features of a Systemic Coronavirus-Associated Disease Resembling Feline Infectious Peritonitis in the Domestic Ferret (Mustela putorius). Vet Pathol. 2008; 45: 236-246.
- Haake C, Cook S, Pusterla N, et al. Coronavirus Infectious in Companion Animal: Virology, Epidemiology, Clinical and Pathologic Features. Viruses. 2020; 12:1023-1043.
- Kipar A and Meli ML. Feline Infectious Peritonitis: Still an Enigma? Veterinary Pathology. 2014; 51(2): 505-526.
- Koharto A, Toba M, Alzoubi A, et al. Formation of Plexiform Lesions in Experimental Severe Pulmonary Arterial Hypertension. Circulation. 2010; 121: 2747-2754.
- Labelle P. The Eye. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th eds. St Louis, MO: Elsevier. 2022; 1432.
- Maclachlan NJ and Dubovi EJ. Coronavirus. In: Maclachlan NJ and Dubovi EJ, eds. Frenner’s Veterinary Virology. 5th eds. Elsevier. 2017: 435-449.
- Miller AD and Porter BF. Nervous System. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th eds. St Louis, MO: Elsevier. 2022; 982-983.
- Palttrinieri S, Giordano A, Stranieri A, et al. Feline infectious peritonitis (FIP) and coronavirus disease 19 (COVID-19): Are they similar? Transbound Emerg Dis. 2021; 68: 1786-1799.
- Pedersen NC. An update on feline infectious peritonitis: Virology and immunopathogenesis. The Veterinary Journal. 2014; 201: 123-132.
- Shigemoto J, Muraoka Y, Wise AG, et al. Two cases of systemic coronavirus-associated disease resembling feline infectious peritonitis in domestic ferrets in Japan. Journal of Exotic Pet Medicine. 2014; 23: 196-200.
- Spagnoli ST and Gelberg HB. Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th eds. St Louis, MO: Elsevier. 2022; 485.
- Stanton JB and Zachary JF. Mechanisms of Microbial Infections. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th eds. St Louis, MO: Elsevier. 2022; 263-264.
- Sula MJM and Lane LV. The Urinary System. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th eds. St Louis, MO: Elsevier. 2022; 755-756.
- Zabka TS, Campbell FE, and Wilson DW. Pulmonary Arteriopathy and Idiopathic Pulmonary Arterial Hypertension. Vet Pathol. 2006; 43: 510-522.