Signalment:
Gross Description:
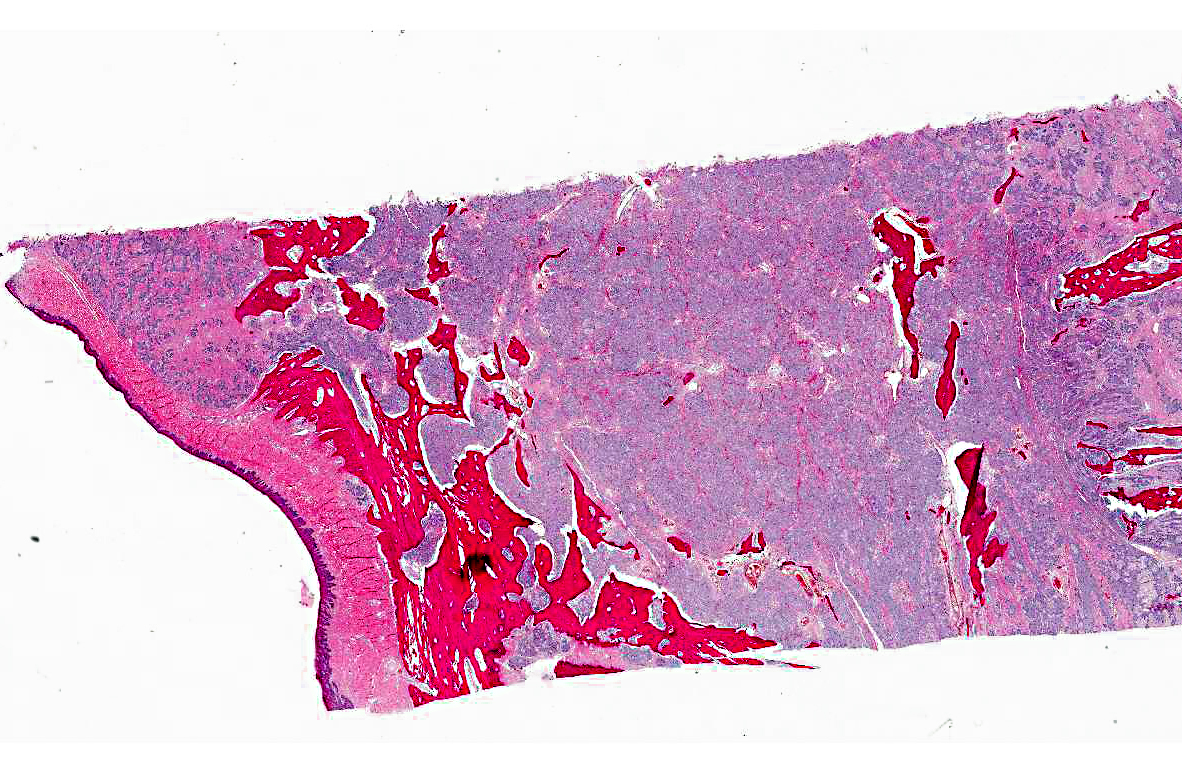
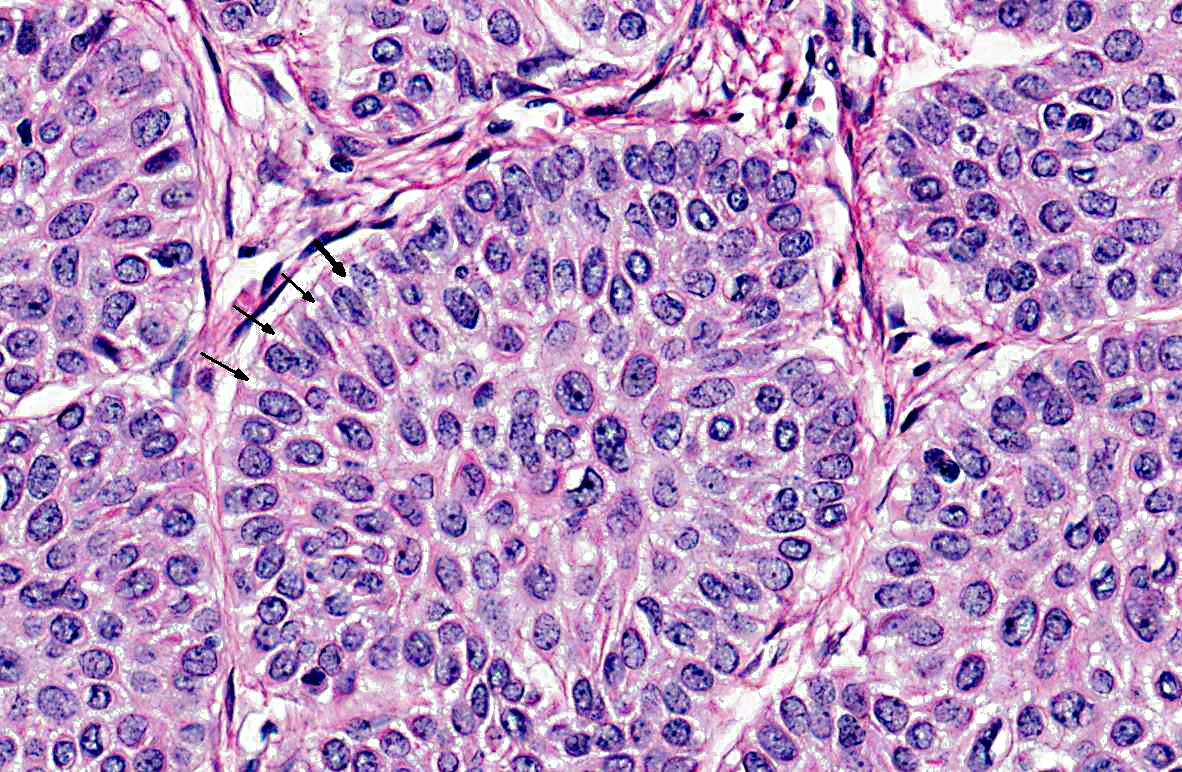
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Another common name for this tumor is acanthomatous epulis although acanthomatous ameloblastoma correctly identifies this tumor as a neoplasia of odontogenic epithelial origin. Epulis is a non-specific, clinical designation used to describe localized, exophytic, nonneoplastic and neoplastic gingival growths.(4,6,7) Epulides are generally classified as fibromatous, ossifying and giant cell types with fibromatous epulides occurring most frequently. Fibromatous, ossifying, and giant cell epulides are thought to be developmental, inflammatory and/or hyperplastic in origin and often develop in association with chronic inflammation (periodontal disease) whereas acanthomatous ameloblastomas are invasive, recurrent and generally occur in animals with milder dental plaque and inflammation. Shetland sheepdogs and mixed breed dogs appear to develop all types of epulides and acanthomatous ameloblastomas. Fibromatous, ossifying and giant cell epulides most commonly develop from the gingiva around the maxillary and mandibular premolars whereas acanthomatous epulides (71%) arise from the gingiva around the maxillary and mandibular canines. Marginal excision is generally curative for fibromatous, ossifying and giant cell epulides (90%) although acanthomatous ameloblastomas persistently exhibit invasive growth, bone infiltration and recurrence following marginal excision.(7)
JPC Diagnosis:
Conference Comment:
Conference participants discussed the variation in odontogenic tumor naming and classification schemes in veterinary and human medicine. In humans, ameloblastomas are classified into several clinicopathologic subtypes: conventional solid or multicystic, unicystic and peripheral.(1) The vast majority of human ameloblastomas are of the conventional solid or multicystic type, which appear grossly as intraosseous growths with both solid and cystic areas. Microscopically the growth pattern of these tumors are divided into the more common follicular and plexiform patterns and the less common acanthomatous, granular cell, desmoplastic, and basal cell patterns. The follicular variants appear as islands of odontogenic epithelium within a mature fibrous stroma, often with cyst formation. The plexiform variant is composed of long anastomosing cords and sheets of odontogenic epithelium, on a more loosely arranged, vascular stroma, with cysts formation much less common. Other, less common variants include the acanthomatous variant, in which squamous differentiation with keratinization or keratin pearl formation occurs within the central regions of the tumor islands, and the granular cell variant, which is composed of cells with abundant eosinophilic, granular cytoplasm within the center of the tumor islands. In the desmoplastic variant the stroma is composed of dense collagen, and the epithelial component is relatively sparse. The basal cell variant closely resembles basal cell carcinomas of the skin; they exhibit nests or islands of basaloid epithelial cells, with less evident peripheral nuclear palisading and reverse polarization and no stellate reticulum. It is possible to have multiple types within the same tumor, and the microscopic diagnosis is made based on the dominant growth pattern. All subtypes of conventional ameloblastomas tend to be locally aggressive and tend to recur with conservative treatment. A second subtype of ameloblastoma in humans is the unicystic ameloblastoma; these tend to occur in younger patients and exhibit less aggressive biological behavior than their conventional counterparts. Microscopically these tumors appear as a single cystic sac. The third subtype, peripheral ameloblastoma, arises within soft tissue, as opposed to the other intraosseous forms. Clinically these tumors may look like a fibroma, granuloma or papilloma. Microscopically, they are similar to conventional ameloblastomas and follicular, plexiform, acanthomatous and basal cells patterns are possible. Odontogenic epithelium is often exhibited but stellate reticulum-like differentiation may not be evident. Neoplastic epithelium may be continuous with overlying surface mucosal epithelium. Peripheral ameloblastomas exhibit a less aggressive behavior and have a lower recurrence rate than their intraosseous counterparts.(1)
Tumors of odontogenic epithelium without odontogenic mesenchyme in animals are classified as peripheral or central ameloblastomas, amyloid-producing odontogenic tumors, or acanthomatous ameloblastomas.(3) Peripheral and central ameloblastomas are defined as tumors occurring from gingival soft tissue or deeper tissue within the bone of the jaw, respectively. Acanthomatous ameloblastomas are more aggressive and can be differentiated from peripheral ameloblastomas by an increased amount of stroma that resembles periodontal ligament connective tissue (characterized by abundant fibrillar collagen, regularly-positioned stellate mesenchymal cells, and regularly dispersed empty blood vessels) and a plexiform pattern consisting of interconnecting cords and sheets of epithelium. Cysts often form in acanthomatous ameloblastomas, but keratinization or keratin pearl formation is rare. Keratinization is more common in ameloblastomas, and heavily keratinized ameloblastomas may be difficult to differentiate from squamous cell carcinoma.(3)
References:
2. Head KW, Else RW, Dubielzig RR. Tumors of the alimentary tract. Canine Acanthomatous Ameloblastoma. In: Tumors in Domestic Animals. 4th ed. Ames, Iowa: Iowa State Press; 2002:405-406.
3. Head KW, et al. Tumors of odontogenic epithelium without odontogenic mesenchyme. In: Tumors of the Alimentary System of Domestic Animals. Washington DC: AFIP and CL Davis DVM Foundation and WHO Collaborating Center for Worldwide Reference on Comparative Oncology; 2003: 49-51.
4. Gardner DG. Epulides in the dog: a review. J Oral Path Med. 1996;25:32-37.
5. Gardner DG, Baker DC. The relationship of the canine acanthomatous epulis to ameloblastoma. J Comp Path. 1993;108:47-55.
6. Verstraets FJM, Ligthelm AJ, Weber A. The histological nature of epulides in dogs. J Comp Path. 1992;106:169-182.
7. Yoshida K, Yanai T, Iwasaki T, Sakai H, Ohta J, Kati S, et al. Clinicopathological study of canine oral epulides. J Vet Med Sci. 1999;61(8):897-902.