Signalment:
Gross Description:
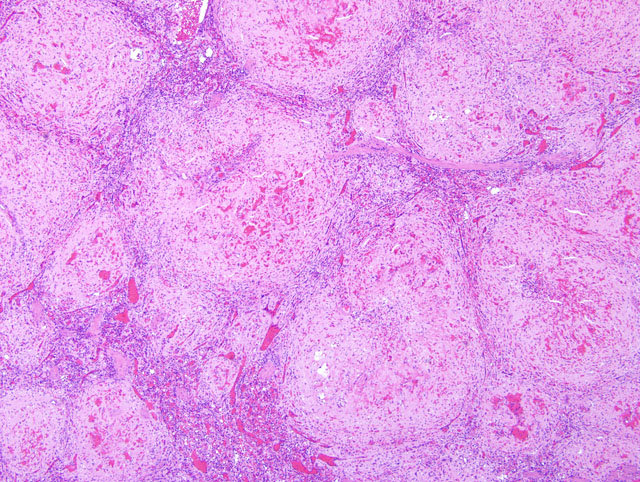
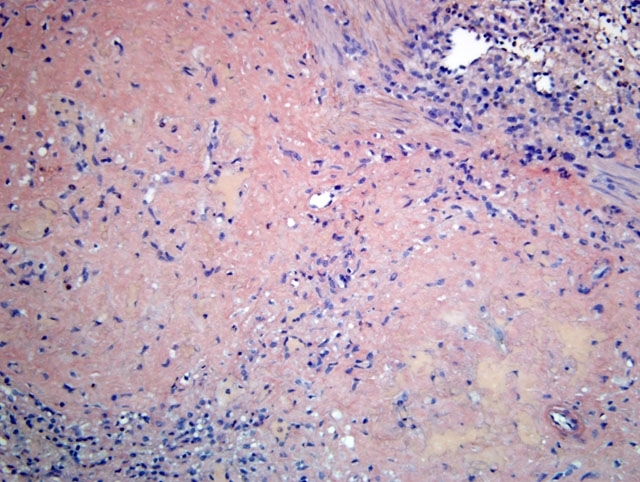
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Amyloidosis may be primary or secondary. Primary amyloidosis arises due to overproduction of the immunoglobulin light chain and may be neoplastic or genetic in origin. Secondary, or reactive systemic amyloidosis is a complication of chronic infections and inflammatory conditions and is characterized by a sustained acute phase response. Although the pathogenesis of reactive systemic amyloidosis is poorly understood, it is associated with persistently increased production of SAA (thus making SAA a major acute phase reactant). Although its major physiological function remains unclear, SAA is produced under the control of numerous cytokines, including interleukin-1, interleukin-6 and tumor necrosis factor-α released during inflammation. Increased levels of SAA are common in chronic inflammation, but amyloid deposition usually does not occur. In individuals that do develop amyloidosis, there is limited or defective SAA proteolysis with formation and deposition of insoluble AA protein. Proposed underlying mechanisms include failure of degradation due to excess levels of SAA relative to enzyme; an intrinsic proteolytic enzyme defect; or a structural defect in the SAA molecule that makes it resistant to degradation. The end result, however, is that accumulated amyloid deposits cause pressure atrophy of surrounding tissues, thus impairing normal body function and resulting in organ failure and ultimately death.
Reactive systemic amyloidosis is not uncommon in rhesus macaques, and has additionally been reported in other nonhuman primates (including common marmosets, squirrel monkeys, pigtail macaques, Celebes macaques, cynomolgus macaques, a stumptailed macaque, baboons, a mangabey and chimpanzees). The condition has been associated with chronic vascular catheterization, as well as underlying conditions such as rheumatoid arthritis, retroviral infection, parasitism, and enterocolitis. Amyloid deposition is most frequently seen in the space of Disse in the liver, the lamina propria of the gastrointestinal tract, the corticomedullary junction of the adrenal gland, either the red or white pulp of the spleen, and the renal medullary interstitium. The small intestine is the region of the gastrointestinal tract most often and most severely affected. Renal glomerular involvement is rare, except in marmosets.
Clinical signs are related to the affected site as well as the amount of amyloid deposited, but include weight loss, diarrhea, and hepatosplenomegaly. Protein losing enteropathy may accompany enteric amyloidosis. Laboratory findings may include elevated levels of SAA, hypoproteinemia, hypoalbuminemia, hypergammaglobulinemia, and elevated liver enzymes with hepatic involvement. There is, however, no reliable diagnostic assay, preventive measure or treatment for secondary amyloidosis. Although gross postmortem lesions are often absent, the liver and/ or spleen may be massively enlarged, pale, waxy and firm. Prominent splenic nodules may be seen cut section, and the intestinal mucosa may be thickened. With the light microscope and hematoxylin and eosin staining, amyloid appears as an amorphous, eosinophilic, extracellular substance. Its differentiation from other similar appearing materials, like fibrin and collagen, depends on the pathognomonic, red-green birefringence observed when preparations correctly stained with Congo red are viewed in intense unidirectional polarized light. This optical effect is produced by alignment of the dye molecules along the protein fibrils.
JPC Diagnosis:
Conference Comment:
| Amyloidosis | |||
|---|---|---|---|
| Category | Associated Diseases | Major Fibril Protein | Precursor Protein |
| Systemic | |||
| Primary amyloidosis (immunocyte dyscrasias) | Multiple myeloma Monoclonal B cell proliferations | AL | Immunoglobulin light chains |
| Secondary amyloidosis (reactive systemic amyloidosis) | Chronic inflammation | AA | SAA |
| Familial amyloidosis (may be systemic and/or localized) | Amyloidosis in Shar-Pei dogs (renal medullary interstitium), Abyssinian cats (renal glomeruli), and Siamese cats (liver) | AA | SAA |
| Localized | |||
| Amyloid of aging | Senile plaques Cerebral amyloid angiopathy Neurodegenerative disease | Aβ | APP |
| Endocrine tumors | Thyroid C cell tumors | A Cal | Calcitonin, polypeptide hormones and/or prohormones |
| Islets of Langerhans | Diabetes mellitus | IAPP | IAPP |
| Isolated amyloid of pulmonary vasculature | Apolipoprotein A-1 | Apolipoprotein A-1 | |
| Prion diseases | Transmissible spongiform encephalopathies | Misfolded prion protein (PrPsc) | Normal prion protein (PrP) |
| AL = amyloid light chain; AA = amyloid associated; SAA = serum amyloid A; APP = amyloid precursor protein; A Cal = amyloid of hormone origin; IAPP = islet amyloid polypeptide | |||
References:
2. Blanchard JL: Generalized amyloidosis, nonhuman primates. In: Monographs on Pathology of Laboratory Animals: Nonhuman Primates I, eds. Jones TC, Mohr U, Hunt RD, pp. 194-197. Springer-Verlag, Berlin Heidelberg, Germany, 1994
3. Cohen AS: General introduction and a brief history of the amyloid fibril. In: Amyloidosis, eds. Marrink J, Van Rijswijk MH, pp. 3-19. Nijhoff, Dordrecht, The Netherlands, 1986
4. Hukkanen RR, Liggitt HD, Anderson DM, Kelley ST: Detection of systemic amyloidosis in the pig-tailed macaque (Macaca nemestrina). Comp Med 56:119-127, 2006
5. Jakob W: Spontaneous amyloidosis of mammals. Vet Pathol 8:292-306, 1971
6. Ludlage E, Murphy CL, Davern SM, Solomon A, Weiss DT, Glenn-Smith D, Dworkin S, Mansfield KG: Systemic AA amyloidosis in the common marmoset. Vet Pathol 42:117-124, 2005
7. MacGuire JG, Christe KL, Yee JL, Kalman-Bowlus AL, Lerche NW: Serologic evaluation of clinical and subclinical secondary hepatic amyloidosis in rhesus macaques (Macaca mulatta). Comp Med 59:168-172, 2009
8. Pepys MB: Amyloidosis. Ann Rev Med 57:223-241, 2006
9. Puchtler H, Sweat F, Levine M: On the binding of Congo red by amyloid. J Histochem Cytochem 10:35564, 1962
10. Snyder PW: Diseases of immunity. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., p. 248. Mosby Elsevier, St. Louis, MO, 2007