WSC 2022-2023
Conference 12
Case II
Signalment:
9-year-old, spayed female, Welsh Corgi dog (Canis lupus familiaris)
History:
The patient presented for lethargy, anorexia, and labored breathing with 2 days duration. On physical examination, the submandibular lymph nodes were enlarged and the lungs sounded harsh on auscultation. Radiographs were unremarkable. The patient’s condition worsened and the patient passed away overnight.
Gross Pathology:
Necropsy was performed by the rDVM and multiple fixed tissue samples were submitted for diagnostic evaluation (necropsy-in-a-bottle). Gross findings were not provided.
Laboratory Results:
Results from a complete blood count, blood chemistry, and T4 on the day of presentation for clinical signs was provided by the rDVM. The following abnormalities were noted:
|
Analyte |
Result |
Reference Range |
Units |
|
WBC |
14.4 |
4.0-15.5 |
103/uL |
|
Neutrophils |
12096 (high) |
2060-10600 |
/uL |
|
Monocytes |
864 (high) |
0-840 |
/uL |
|
ALP |
256 (high) |
5-131 |
IU/L |
|
BUN |
187 (high) |
6-31 |
mg/dL |
|
Creatinine |
11.4 (high) |
0.5-1.6 |
mg/dL |
|
Phosphorus |
23.6 (high) |
2.5-6.0 |
mg/dL |
|
Glucose |
65 (low) |
70-138 |
mg/dL |
|
Calcium |
13.0 (high) |
8.9-11.4 |
mg/dL |
|
Corrected Ca |
13.2 |
|
|
|
Magnesium |
3.3 (high) |
1.5-2.5 |
mEq/L |
|
Sodium |
143 |
139-154 |
mEq/L |
|
Potassium |
7.2 (high) |
3.6-5.5 |
mEq/L |
|
Na/K ratio |
20 (low) |
27-38 |
|
|
Amylase |
1145 (high) |
290-1125 |
IU/L |
|
PrecisionPSL |
364 (high) |
24-140 |
U/L |
|
T4 |
<0.5 (low) |
0.8-3.5 |
ug/dL |
Microscopic Description:
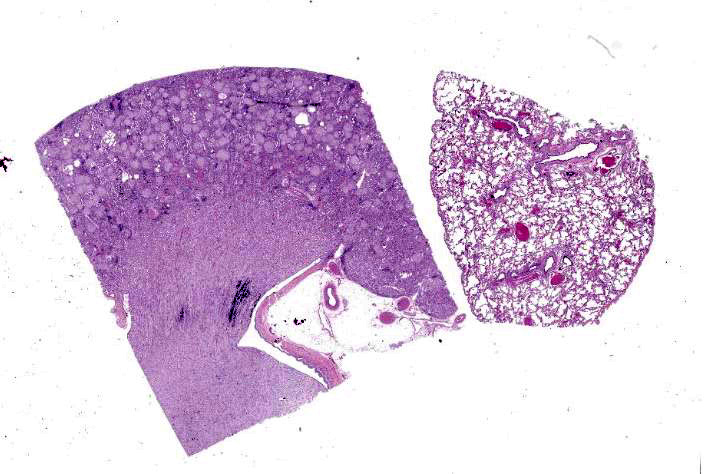
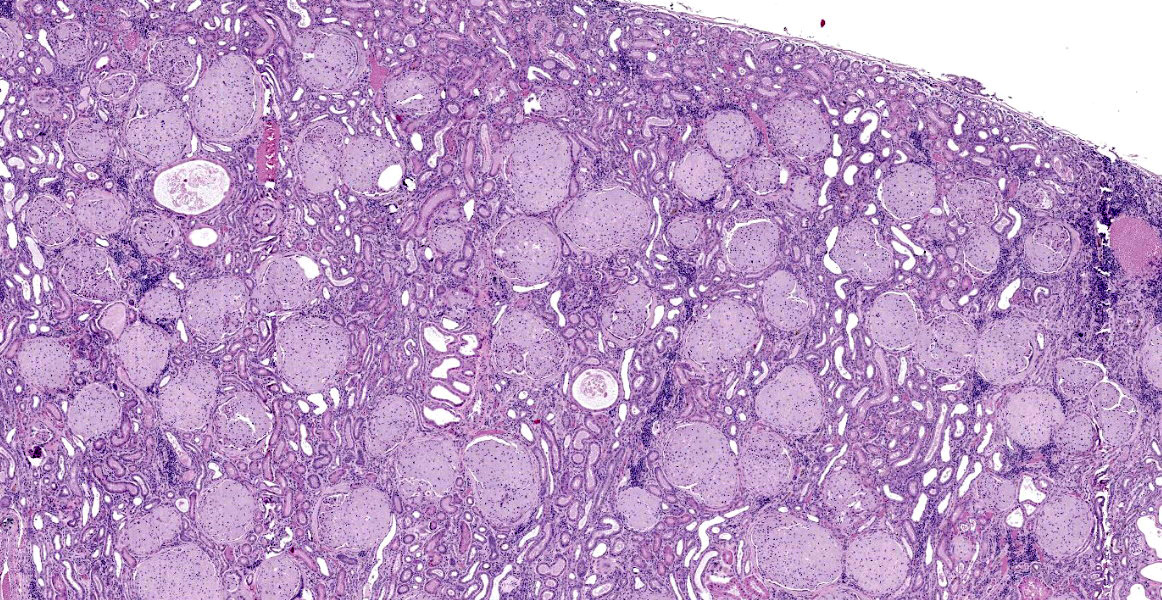
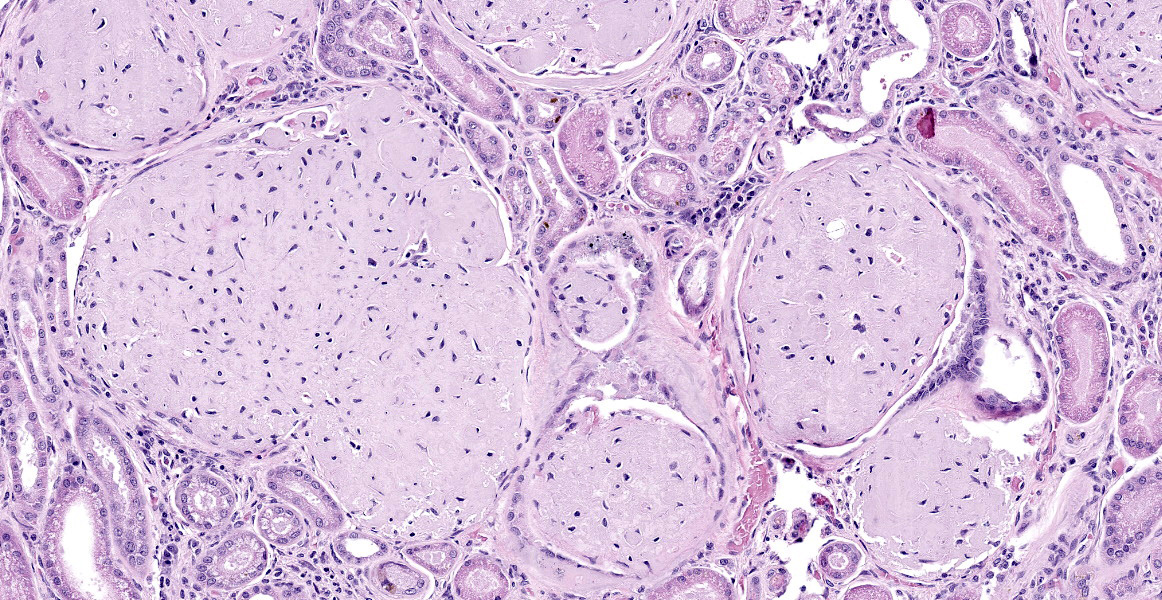
Kidney: The glomeruli are markedly, diffusely, and globally enlarged by pale, smudgy to amorphous, faintly fibrillar, extracellular eosinophilic material expanding the mesangial matrix and obliterating the glomerular tufts. Glomerular capillaries are compressed, and the glomerular architecture is obscured by the extracellular deposits. Bowman’s capsule is occasionally and mildly thickened by similar material and rarely surrounded by a few concentric thin bands of eosinophilic fibrous connective tissue (fibrosis). Renal tubules are characterized by various changes, including: 1) lumina distended by clear space and lined by normal epithelium, 2) lumina containing granular debris and lined by markedly flattened (attenuated) epithelium mixed with a few angular and hypereosinophilic epithelial cells, 3) thinned tubules lined by plump epithelial cells with an open nucleus (regeneration), and 4) epithelial cells that are mildly vacuolated and contain numerous small eosinophilic protein droplets. The interstitium is multifocally infiltrated by small to modest numbers of lymphocytes and plasma cells. While less distinct and severe, the medullary interstitium is mildly expanded by similar eosinophilic material described in the glomeruli.
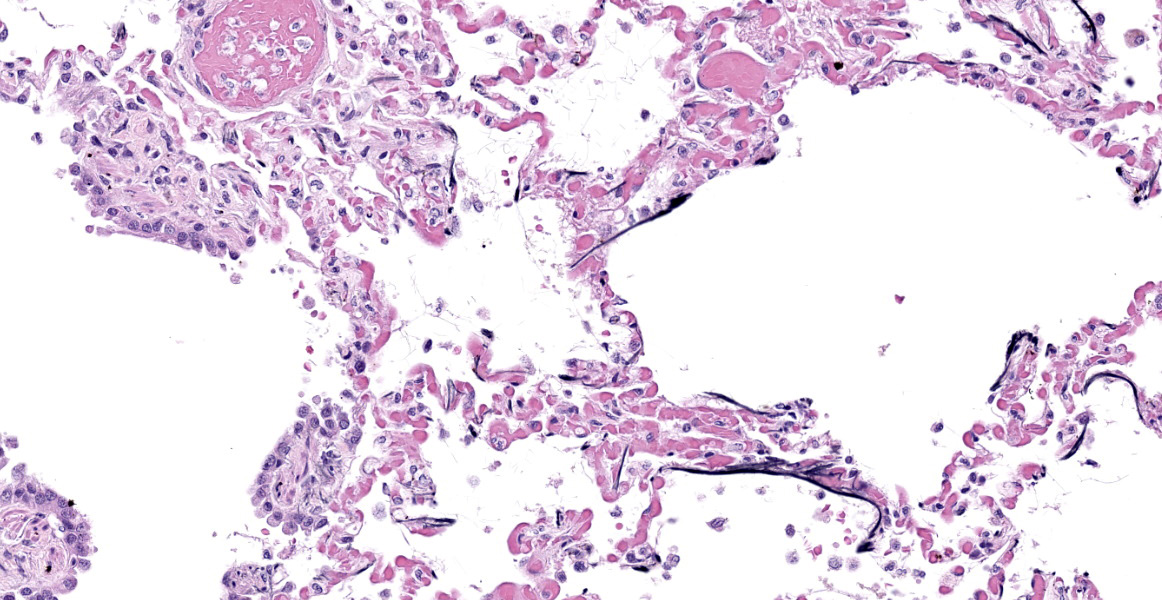
Lung: Alveolar and parenchymal vessels are diffusely congested. The alveolar spaces are often mildly expanded by clear space and contain low numbers of extravasated erythrocytes and fine fibrillary material. In some areas, there is a very faint, thin blue and granular hue to the lining of the alveolar septa (mineralization).
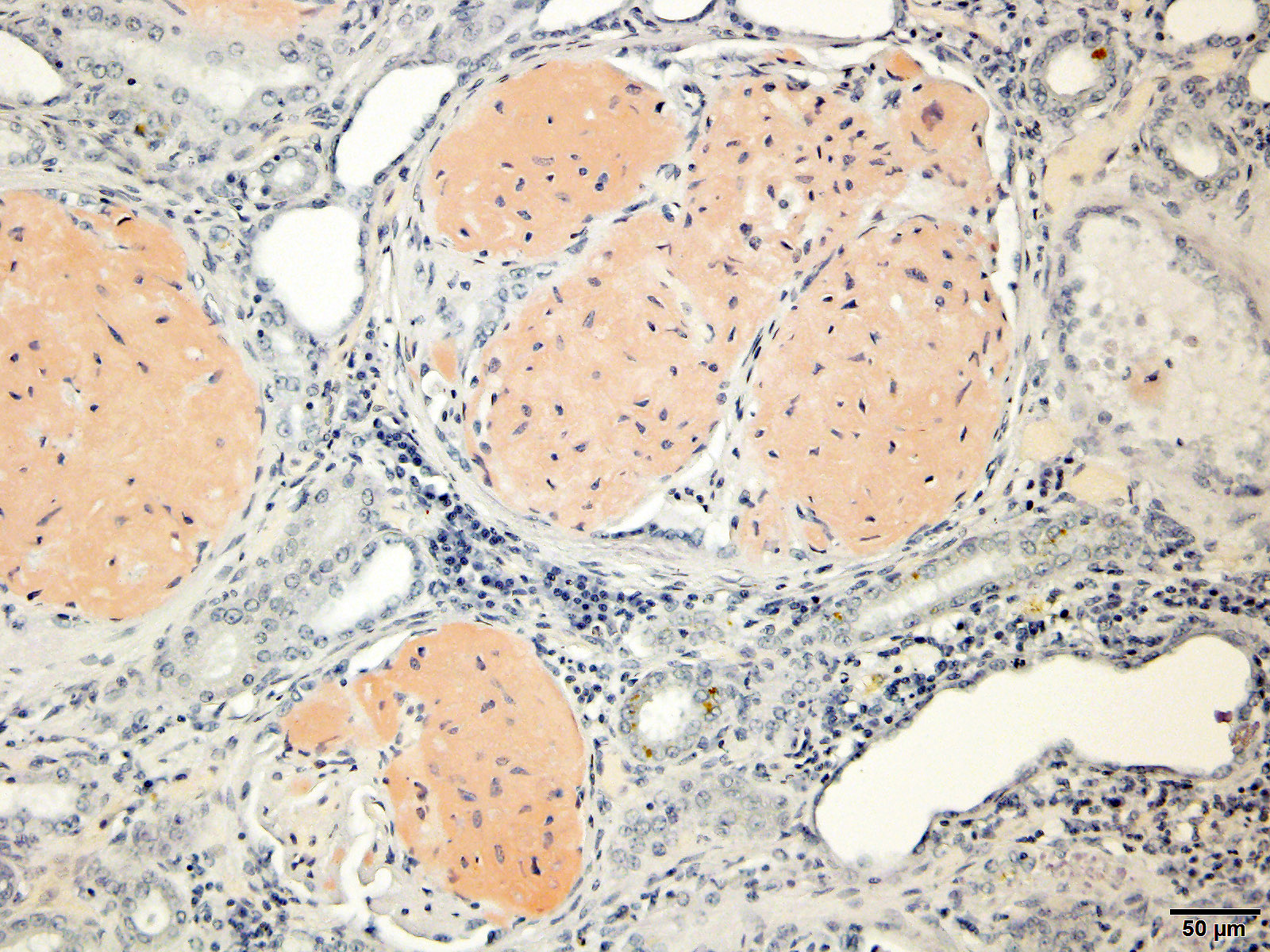
Special staining of the kidney with Congo Red and the lung with von Kossa is performed. Within the kidney, the glomeruli are expanded by orange to red material that rarely exhibits apple-green birefringence under polarized light. The presence of Congophilic material expanding the glomeruli is consistent with amyloid. Within the lung, multifocally lining alveolar septa and partially lining small-caliber parenchymal vessels are thin linear bands of material that stain black, consistent with mineral.
Contributor’s Morphologic Diagnoses:
Kidney: Severe glomerular amyloidosis with mild renal tubular degeneration and atrophy, and lymphoplasmacytic interstitial nephritis with fibrosis
Lung: Acute pulmonary congestion with alveolar mineralization and damage
Contributor’s Comment:
Amyloidosis is a disease condition that results when amyloid, a proteinaceous material, is deposited intercellularly in a variety of tissues, including the kidneys.3 Amyloid refers to a group of insoluble, fibrillary proteins with diverse origins but similar structures and properties. 10
Three of the most prominent forms of amyloidosis in animals include reactive, immunoglobulin-derived, and familial amyloidosis. The most common form of amyloidosis is reactive systemic amyloidosis, also called secondary amyloidosis. It results from AA-amyloid being derived from serum amyloid A (SAA), an acute-phase lipoprotein. AA-amyloidosis is often associated with chronic inflammatory disease, persistent infections, or neoplasia. Immunoglobulin-derived amyloidosis, also called primary amyloidosis, is the most common form in humans but less common in domestic animals. It involves AL-amyloid, which is derived from immunoglobulin light chains. 3,10 Familial amyloidosis is another form of amyloidosis that is most commonly seen in Shar Pei dogs and Abyssinian cats.9
Amyloidosis occurs when there is misfolding of the progenitor protein. 10 Amyloid proteins are arranged in a β-pleated sheet structure. This renders amyloid insoluble and resistant to proteolysis, allowing it to permanently accumulate in tissues.7 In the case of AA-amyloidosis, increased SAA due to inflammation coupled with a defect in SAA degradation result in the accumulation of misfolded protein fibrils. 10 Amyloid damages tissues by causing pressure atrophy to adjacent cells. 3 The most common site of amyloid accumulation in dogs is the kidney where it usually affects glomeruli.9 Amyloid is often also deposited in the liver, spleen, lymph nodes, and adrenal glands. 10
Gross changes associated with renal amyloidosis include mild renomegaly, pallor, and a waxy consistency. 3 Microscopically, amyloid is deposited extracellularly.10 In the kidneys, these deposits are within the mesangial area of the glomerulus.3 This results in expansion of the mesangium, compressing the adjacent capillary loop.4 As amyloid accumulates, the glomeruli become enlarged and homogeneous in appearance.3 In the case of familial amyloidosis in Shar Pei dogs, amyloid often accumulates in the renal medullary interstitium.9 In order to differentiate amyloid from other extracellular deposits, Congo red stain is used. Congo red stains amyloid deposits orange to red with apple-green birefringence under polarized light.10
As renal amyloidosis is irreversible, it often leads to chronic kidney failure. In this case, the dog’s serum biochemistry profile indicated severe azotemia, consistent with kidney disease. Additional biochemical findings in this case that are likely attributed to chronic renal failure include hyperkalemia and hyperphosphatemia. Other clinicopathologic abnormalities of renal failure that were either not reported or normal in this case included non-regenerative anemia and metabolic acidosis. Importantly, results of a urinalysis were not provided in this case and so proteinuria, a hallmark of renal amyloidosis, in addition to other common urinalysis findings seen with chronic renal disease cannot be excluded.
A common finding associated with chronic kidney failure and renal azotemia is alveolar mineralization within the lungs.6 Often called uremic pneumonopathy, mineralization occurs within the pulmonary interstitium, specifically the smooth muscle and connective tissue fibers of alveolar septa, veins, and bronchioles.2 Although uremic mineralization can be multisystemic, mineral deposition often occurs in sites affected by degenerative or necrotic tissue damage. Due to this characteristic and since uremic mineralization can occur regardless of serum calcium concentration, it is most consistent with dystrophic mineralization, as opposed to metastatic mineralization.1 Other alveolar changes associated with mineralization include pulmonary edema and histiocytosis. Dogs with mineralization of alveoli may or may not exhibit clinical signs of respiratory disease.2
Contributing Institution:
University of Illinois at Urbana-Champaign, Veterinary Diagnostic Laboratory
http://vetmed.illinois.edu/vet-resources/veterinary-diagnostic-laboratory/
JPC Diagnosis:
- Kidney: Amyloidosis, glomerular, global, diffuse, severe, with tubular degeneration, proteinosis, and chronic lymphocytic interstitial nephritis.
- Lung: Mineralization, subpleural, septal, and vascular, multifocal, moderate.
JPC Comment:
The contributor provides an excellent and succinct comment in this classic condition. Amyloidosis is a subject of frequent research due to its prevalence, diverse presentations, and varied composition in multiple species, including humans. Differentiating the type of amyloid being deposited is critical for understanding the pathogenesis. Traditionally, light-chain amyloidosis (AL) has been differentiated from AA using special stains: AL retains congophilia when pretreated with potassium permanganate, while AA loses congphilia.5 An additional method to identify AL is immunohistochemical staining for kappa and lambda light chains.5 Recently, laser microdissection-liquid chromatography-tandem mass spectrometry (LMD-MS) has been used to identify the amyloid precursor proteins in humans, and a study in dogs and cats identified immunoglobulin light chains in 17 of 17 extramedullary plasma cell tumors with amyloid deposition.5 In the same study, only 12 of 17 cases showed potassium permanganate resistant congophilia, and light chains were only detected in 6 of 17 cases.5 This study demonstrated that LMD-MS may be more sensitive than traditional methods for identifying light-chain amyloidosis.5
Amyloid signature proteins (ASPs) are proteins that are deposited with amyloid fibrils and are likely involved in the pathogenesis of amyloidosis.8 ASPs studied in man include serum amyloid P component, which protects amyloid fibrils from proteolysis, and various apolipoproteins, including ApoE, which affects the stability and deposition of beta-amyloid.8 ASPs have yet to be studied thoroughly in veterinary species. Recently, immunohistochemistry and LMD-MS were used to investigate which ASPs are present in three types of amyloidosis in cats; ApoE was identified using both methods in 16 of 16 cases, while SAP was not identified in any cases.8 In the previously mentioned study of extramedullary plasma cell tumors, IHC appeared to be more sensitive than LMD-MS, which only identified ASPs in 10 of 15 dogs.5
References:
- Cardoso PGS, Pinto MPR, Moroz LR, et al. Dystrophic Mineralization in Uremic Dogs: An Update. Pesqisa Veterinaria Brasileira. 2019;39: 889-899.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. Philadelphia, PA: Elsevier Saunders. 2016: 494
- Cianciolo RE, Mohr FC. Urinary System. In: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. Philadelphia, PA: Elsevier Saunders. 2016: 413-415.
- Cianciolo RE, Mohr FC, Aresu L, et al. World Small Animal Veterinary Association Renal Pathology Initiative: Classification of Glomerular Diseases in Dogs. Veterinary Pathology. 2016;53: 113-135.
- Kadota A, Iwaide S, Miyazaki S, et al. Pathology and Proteomics=Based Diagnosis of Localized Light-Chain Amyloidosis in Dogs and Cats. Vet Pathol. 2020: 57(5); 658-665.
- Le Boedec K, Heng HG, Snyder PW, Pressler BM. Pulmonary Abnormalities in Dogs with Renal Azotemia. J Vet Intern Med. 2012;26: 1099-1106.
- Mason NJ, Day MJ. Renal Amyloidosis in Related English Foxhounds. Journal of Small Animal Practice. 1996;37: 255-260.
- Miyazaki S, Kadota A, Mitsui I, Murakami T. Amyloid Signature Proteins in Feline Amyloidosis. J Comp Path. 2020; 117:10-17.
- Segev G, Cowgill LD, Jessen S, Berkowitz A, Mohr CF, Aroch I. Renal Amyloidosis in Dogs: A Retrospective Study of 91 Cases with Comparison of the Disease between Shar-Pei and Non-Shar-Pei Dogs. J Vet Intern Med. 2012;26: 259-268.
- Woldemeskel M. A Concise Review of Amyloidosis in Animals. Veterinary Medicine International. 2012; 2012: 427296.