Signalment:
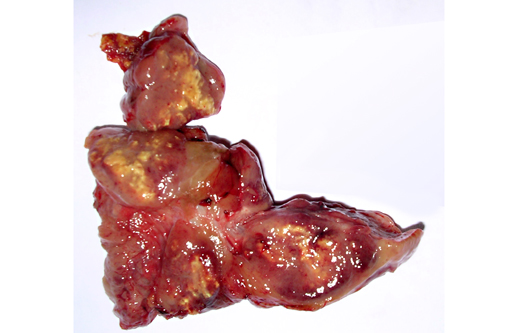
Gross Description:
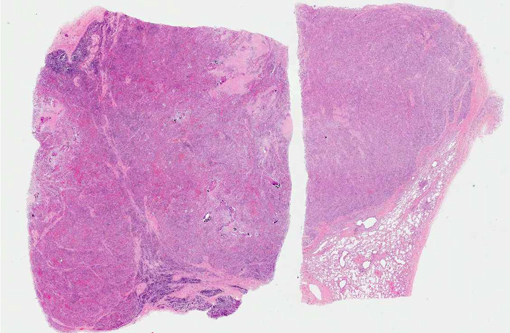
Histopathologic Description:
Lymph node: Granulomatous lesions are similar to those described in the lung.
Morphologic Diagnosis:
Lung: Pneumonia, granulomatous, multifocal to coalescing, chronic, severe, with intra- and extra-histiocytic acid-fast bacilli.Â
Lymph node: Lymphadenitis, granulomatous, multifocal to coalescing, chronic, severe, with intra- and extra-histiocytic acid-fast bacilli.
Lab Results:
Condition:
Contributor Comment:
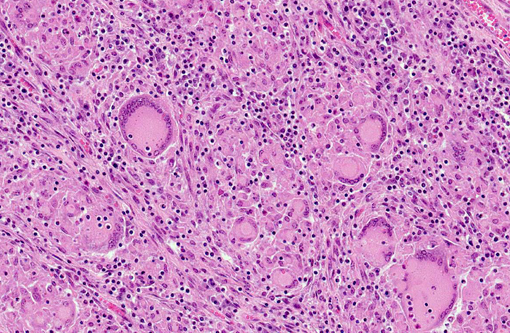
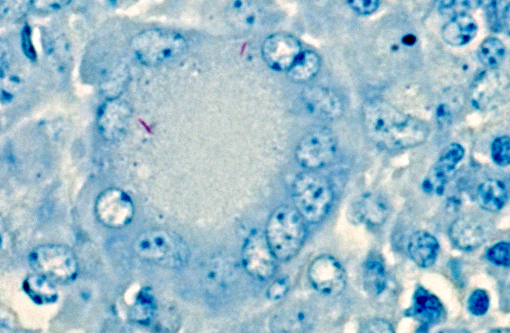
Microscopic lesions consistent with tuberculosis in cattle are often multicentric and coalescing with central areas of caseous necrosis and mineral. Epithelioid macrophages surround the necrosis and often included are small to moderate numbers of multinucleated giant cells with smaller numbers of lymphocytes, plasma cells, and occasional neutrophils.(2) The typical tuberculous lesion caused by Mycobacterium bovis has small to occasionally moderate numbers of acid fast bacteria present within the cytoplasm of macrophages and giant cells as well as areas of necrosis.(2)
Bovine cases fitting this description undergo additional testing using PCR on formalin-fixed, paraffin embedded (FFPE) tissue to test for mycobacterial DNA. Because the scope of the TB eradication program is focused on identifying M. bovis, primers used in the PCR are limited to those for M. tuberculosis complex (MTBC, of which M. bovis is a member), M. avium complex (MAC, common environmental mycobacteria) and M. avium subsp. paratuberculosis (MAP, the organism that causes Johnes disease in cattle). A recent report(12) of mycobacteria cultured from clinical samples submitted to the NVSL stated that the majority of mycobacteria cultured from cattle were M. bovis (32%) followed by M. avium complex (25.5%). The next most common species, M. fortuitum/M. fortuitum complex, comprised 10.1%.(12)
The microscopic features of the current case were consistent with bovine tuberculosis. FFPE tissue was tested by PCR for mycobacterial DNA using our primers for MTBC, MAC, and MAP, and tests were negative. False negative results for mycobacterial DNA can occur for a couple of reasons. First, formalin fixation causes irreversible cross-linking between DNA and protein, which increases as the tissue fixes over time. Over-fixation in formalin can reduce availability of DNA for the PCR, and result in a false negative finding.(5) Tuberculosis-suspect submissions from FSIS are shipped to the NVSL overnight; samples are cut-in immediately upon receipt and processed overnight for microscopic exam the next day. Because this case was an FSIS submission, over-fixation is unlikely as a cause for the false-negative PCR results. Second, false negative results may occur when there are extremely low numbers of AFB, as there were in this case.
M. avium complex was isolated from the tissues of this animal. Further subspeciation of the isolate was not performed. Culture is the gold standard for definitive diagnosis of bovine tuberculosis(1) and takes up to 10 weeks to complete with slow-growing mycobacteria.
Members of the M. avium complex are slow growing saprophytes commonly found in water, soil, and decaying vegetation.(4,6) MAC can cause tuberculosis-like disease in humans (particularly those who are immunocompromised) and birds.(4) Additionally, naturally occurring MAC infections have been reported in a tiger,(3) dogs,(7,9) pigs,(8) and a ferret with lymphoma.(10) Acid-fast bacilli are frequently numerous in lesions caused by MAC organisms,(6) but were uncommonly sparse in this case.
JPC Diagnosis:
Conference Comment:
Mycobacteria are broadly characterized as obligate and opportunistic pathogens. Obligate pathogens include the tuberculosis complex (MTBC: M. tuberculosis and M. bovis) and the leprosy group (M. leprae and M. lepraemurium), while opportunistic mycobacteria are subdivided into rapid-growing (e.g., M. fortuitum, M. chelonae and M. smegmatis) and slow-growing (e.g., M. avium complex (MAC)) mycobacteria.(1) Another classic taxonomic division, which excludes the tuberculosis complex, is the Runyon system. This divides mycobacteria into four groups based on pigment and growth rate; these are summarized in Table 1. Mycobacteria have a protective lipid-rich cell wall with mycolic acid; once phagocytized, they inhibit phagosome-lysosome fusion, thus preventing oxygen radical formation, disrupting cytokine production, and avoiding proteolytic enzymes produced by the host cell.(8)
Some species, such as MTBC in cattle and M. avium subsp. avium in birds, generally produce a TH1 (tuberculoid) reaction, which results in the formation of granulomas with low numbers of AFB (i.e., paucibacillary granulomas). The immunologic basis for this reaction is delayed-type (i.e., type IV) hypersensitivity, with a TH1-type, or cell mediated lymphocytic response; in cattle, this often begins in the lungs, since the respiratory tract is the most common portal of entry for MTBC. Specifically, antigen presenting cells release IL-12, which induces na+�-�ve CD4+ T-lymphocytes to enter the TH1 pathway. Once committed, TH1 cells synthesize and release IL-2, which activates additional TH1-lymphocytes; IFN-γ and TNF-β, which activates and attracts macrophages; and TNF-α, which promotes an inflammatory response. Interferon-γ also inhibits activation of the TH2 pathway.(1) This TH1 response results in classical macrophage activation, which influences the structure of the chronic inflammatory response. Specifically, classical macrophage activation via IFN-γ triggers the expression of MHC II, respiratory burst, and release of NO as well as the cytokines IL-1, IL-6 and TNF. The end result is microbial killing, granuloma formation, cellular immunity and delayed type hypersensitivity,(1) which accounts for the microscopic features observed in this case.
In contrast, some types of mycobacteriosis are characterized by a TH2 (lepromatous) response; examples include leprosy (M. leprae) and Johnes disease (MAP). Here, commitment to the TH2 immune response is induced by IL-4, and TH2-lymphocytes release IL-4, IL-5, IL-10, IL-13, IL-17 and IL-19, which result in B-lymphocyte activation, antibody production (humoral immunity) and alternative macrophage activation. This is an ineffective method of killing mycobacteria, so histologically there is a multibacillary, disseminated granulomatous response, generally in the gastrointestinal tract and mesenteric lymph nodes.(1)
Although MAC can produce both multibacillary, lepromatous lesions and paucibacillary, tuberculoid lesions,(4) M. bovis is more commonly isolated from bovine pulmonary granulomas, so it is somewhat surprising that M. avium was isolated from the submitted tissue and that PCR for MTBC was negative. As the contributor noted, the gross and microscopic features of this case were more consistent with bovine tuberculosis.Â
Table 1: Runyan system of myobacterial classification11
| I | slow-growing photochromogens that turn yellow when exposed to light |
| II | slow-growing scotochromogens that appear yellow in the dark and after exposure to light |
| III | slow-growing non-photochromogens are non-pigmented |
| IV | rapid growers, which show visible growth within seven days |
References:
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Vol. 2. St. Louis, MO: Saunders Elsevier; 2007:523-653.
3. Cho HS, Kim YH, Park NY. Disseminated mycobacteriosis due to Mycobacterium avium in captive Bengal tiger (Panthera tigris). J Vet Diagn Invest. 2006;18:312-314.
4. Coelho AC, Pinto MdL, Matos A, Matos M, Pires MA. Mycobacterium avium complex in domestic and wild animals. Insights from Veterinary Medicine. 2013:91-128.Â
5. Fang SG, Wan QH, Fujihara N. Formalin removal from archival tissue by critical point drying. Biotechniques. 2002;33:604-611.
6. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Vol. 1. St. Louis, MO: Saunders Elsevier; 2007:553-781.
7. Gow AG, Gow DJ. Disseminated Mycobacterium avium complex infection in a dog. Vet Rec. 2008;162:594-595.
8. Hibiya K, Kasumi Y, Sugawara I, Fujita J. Histopathological classification of systemic Mycobacterium avium complex infections in slaughtered domestic pigs. Comp Immunol Microbiol Infect Dis. 2008;31:347-366.
9. O'Toole D, Tharp S, Thomsen BV, Tan E, Payeur JB. Fatal mycobacteriosis with hepatosplenomegaly in a young dog due to Mycobacterium avium. J Vet Diagn Invest. 2005;17:200-204.
10. Saunders GK, Thomsen BV. Lymphoma and Mycobacterium avium infection in a ferret (Mustela putorius furo). J Vet Diagn Invest. 2006;18:513-515.
11. Stahl DA, Urbance JW. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990;172(1):116-124.
12. Thacker T, Robbe-Austerman S, Harris B, Palmer MV, Waters WR. Isolation of mycobacteria from clinical samples collected in the United States from 2004 to 2011. BMC Vet Res. 2013;9:100.