Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 3
September 6th, 2017
CASE IV: B17-798 (JPC 4101575).
Signalment: 14-year-old, spayed, female, Domestic shorthair cat (Felis catus).
History: The owner identified an approximately 1cm nodule associated with the right mammary chain in December. By the following May, it had grown to approximately 4cm and became ulcerated. The right mammary chain and left inguinal mammary gland were removed surgically, and the tissue was submitted for histopathology. At the time of surgery, a single, approximately 4mm pulmonary nodule was identified on thoracic radiographs. On follow-up radiographs one month later, this nodule had enlarged and additional pulmonary nodules were identified.
Gross Pathology: The right inguinal mammary gland was expanded by a 3.7 x 3.2 x 2.8 cm firm, ulcerated mass. The right superficial inguinal lymph node was included in the sample and was also enlarged and firm.
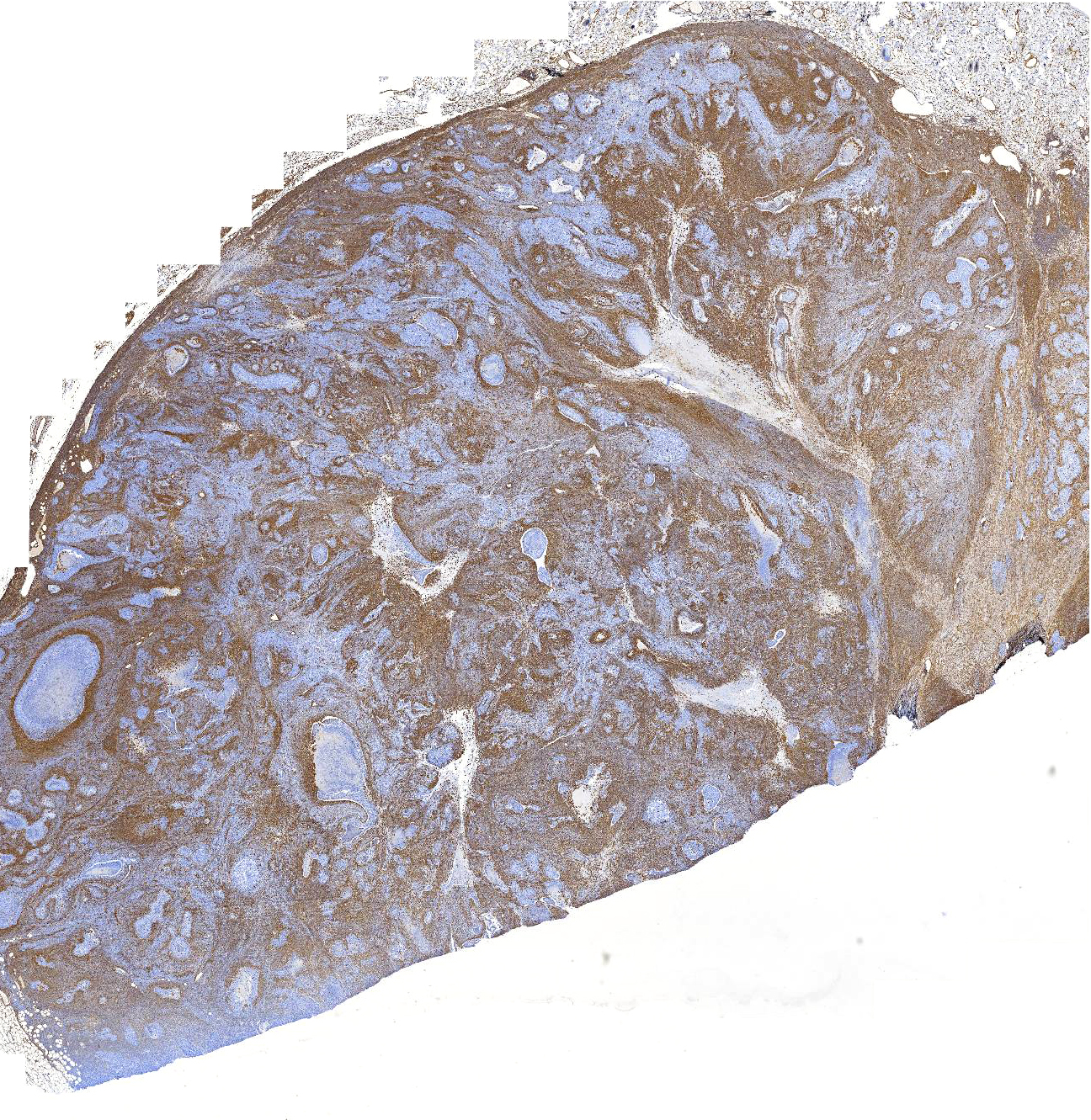
Laboratory results: Immunohistochemistry for cytokeratin, vimentin, and smooth muscle actin was performed to characterize the neoplastic population.
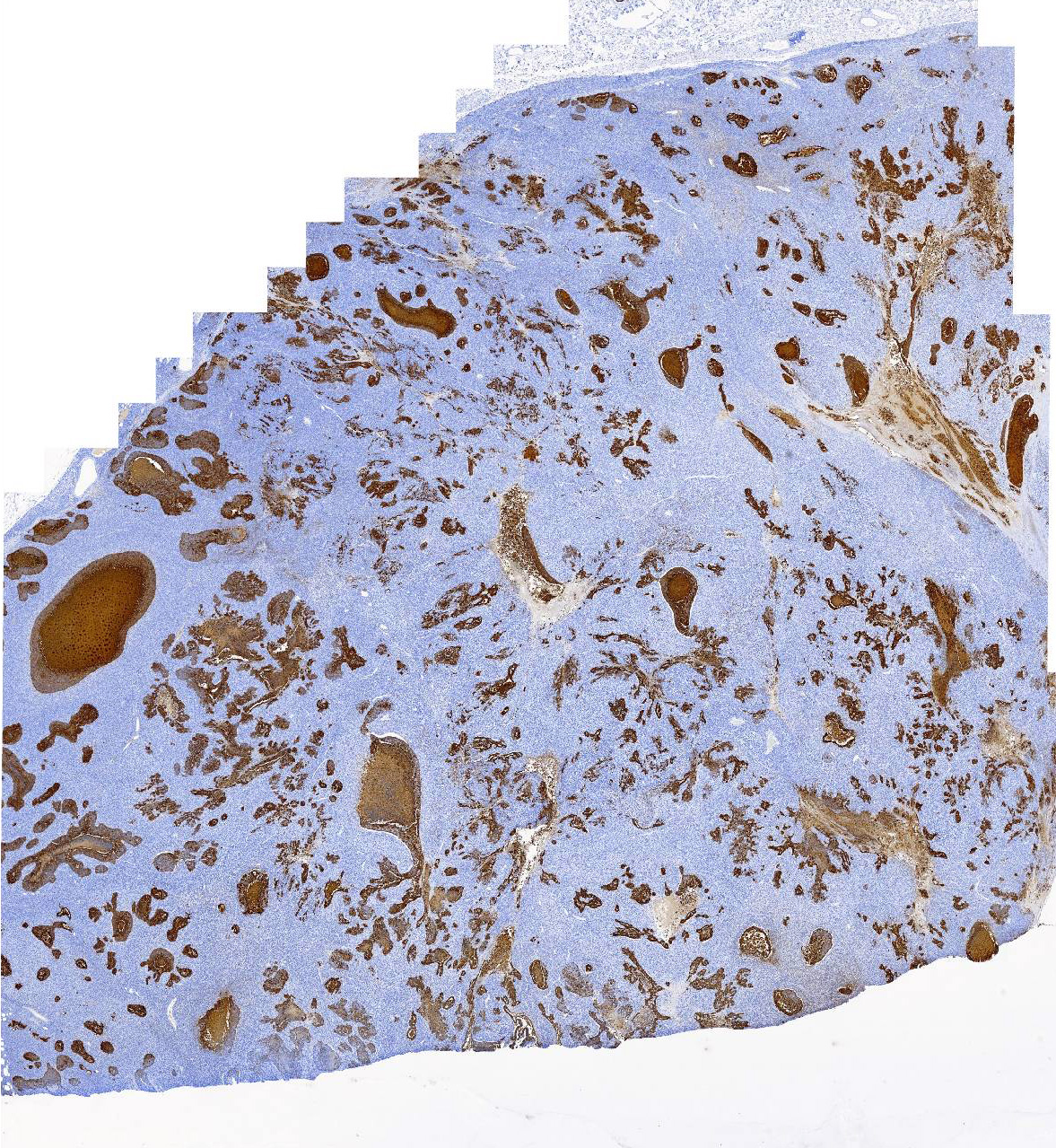
IHC for cytokeratin (AE1/AE3) revealed diffuse, strong, cytoplasmic labeling of the polygonal neoplastic population. Rarely, patchy areas of spindle cells exhibit weak to moderate cytoplasmic labeling (<1% overall).
IHC for vimentin strongly stained the cytoplasm of the majority (approximately 75%) of the neoplastic spindle cells and occasional polygonal cells (<5% overall).
IHC for smooth muscle actin highlights vascular smooth muscle within and around the neoplasm and rarely individual or small groups of neoplastic cells in the epithelial or spindle cell populations (overall <1% of either population).
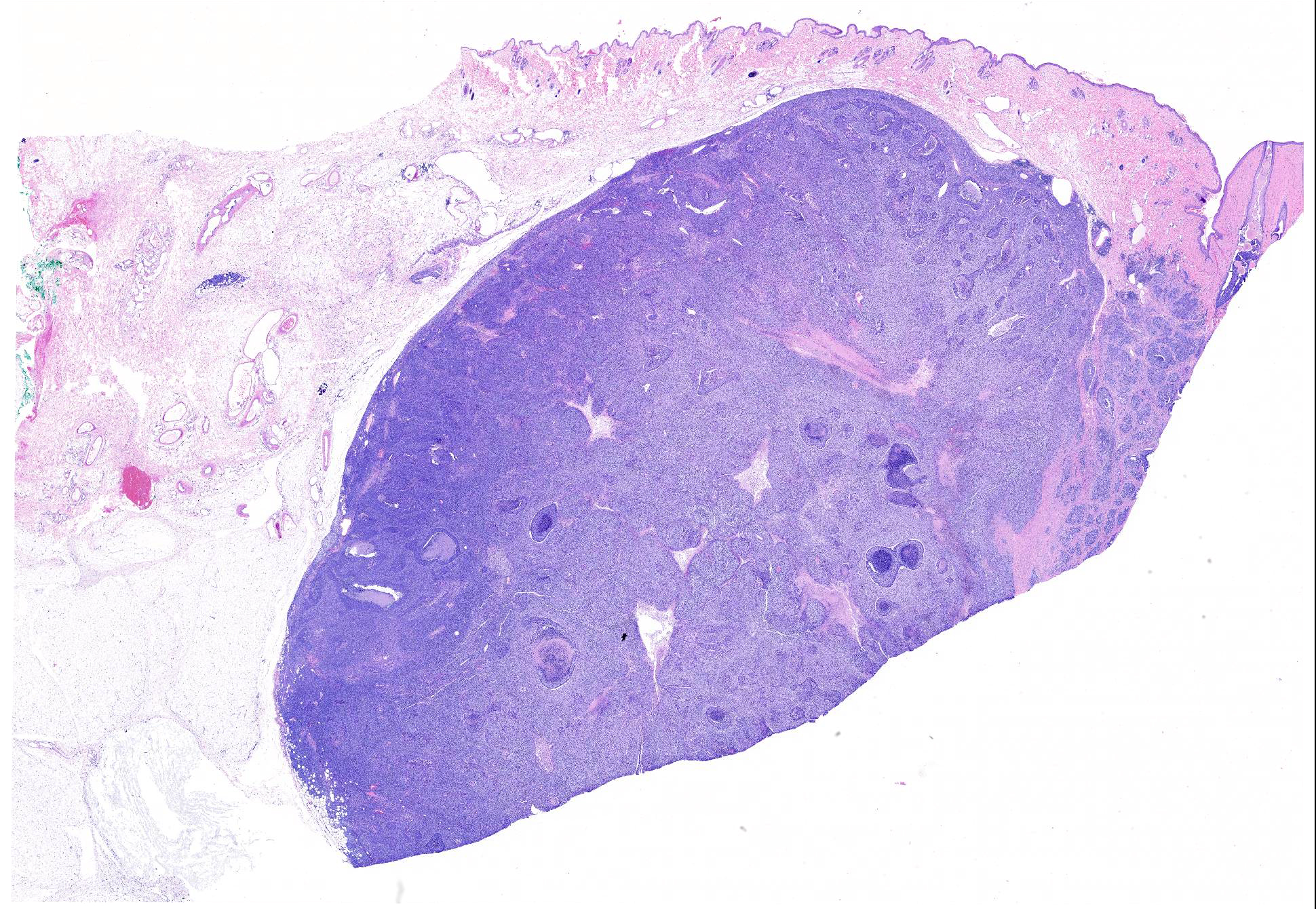
Microscopic Description: Mammary gland with nipple: Expanding and infiltrating the dermis and subcutis is a neoplasm composed of malignant epithelial and spindle cell populations that are typically but not always closely associated with each other.
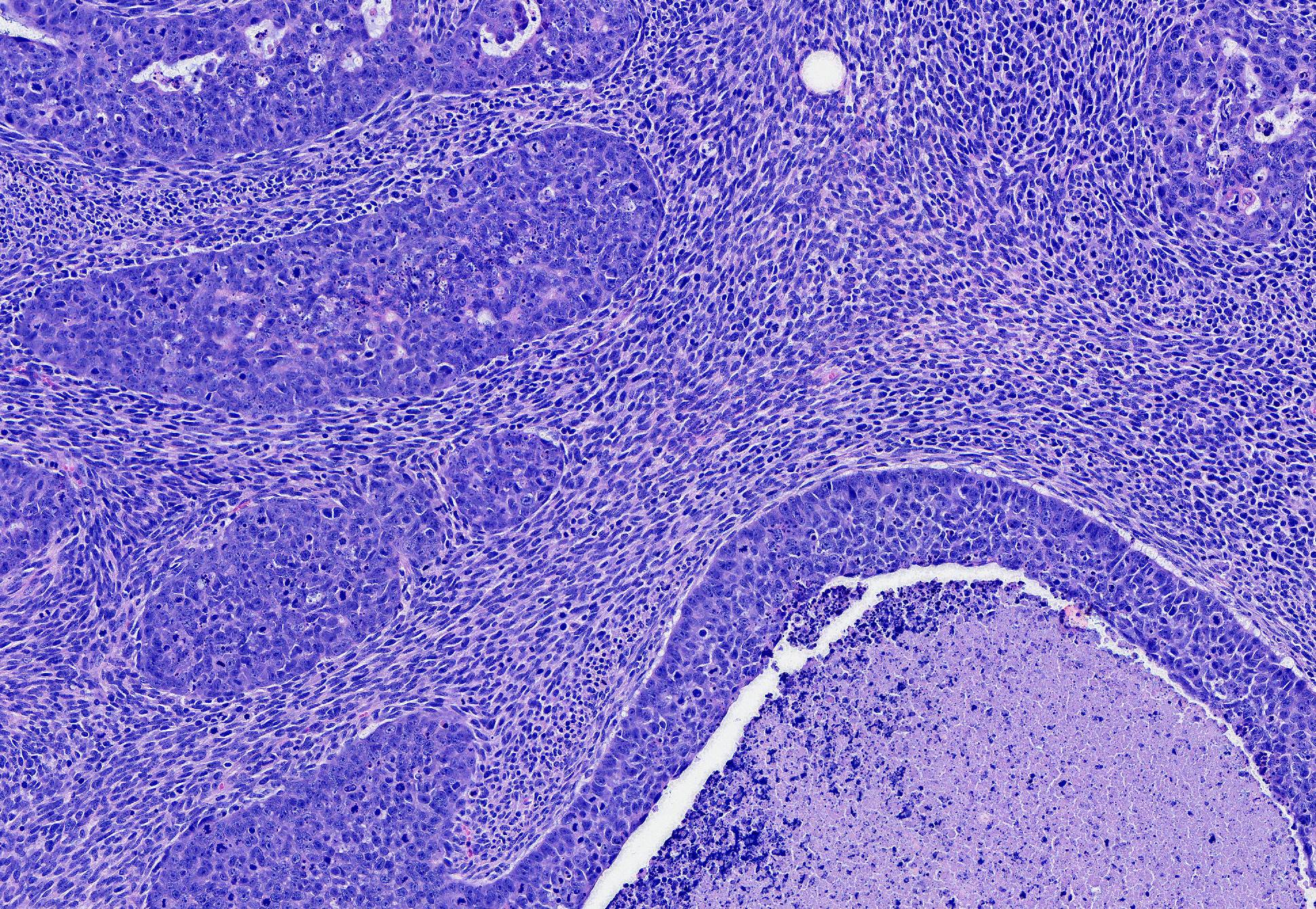
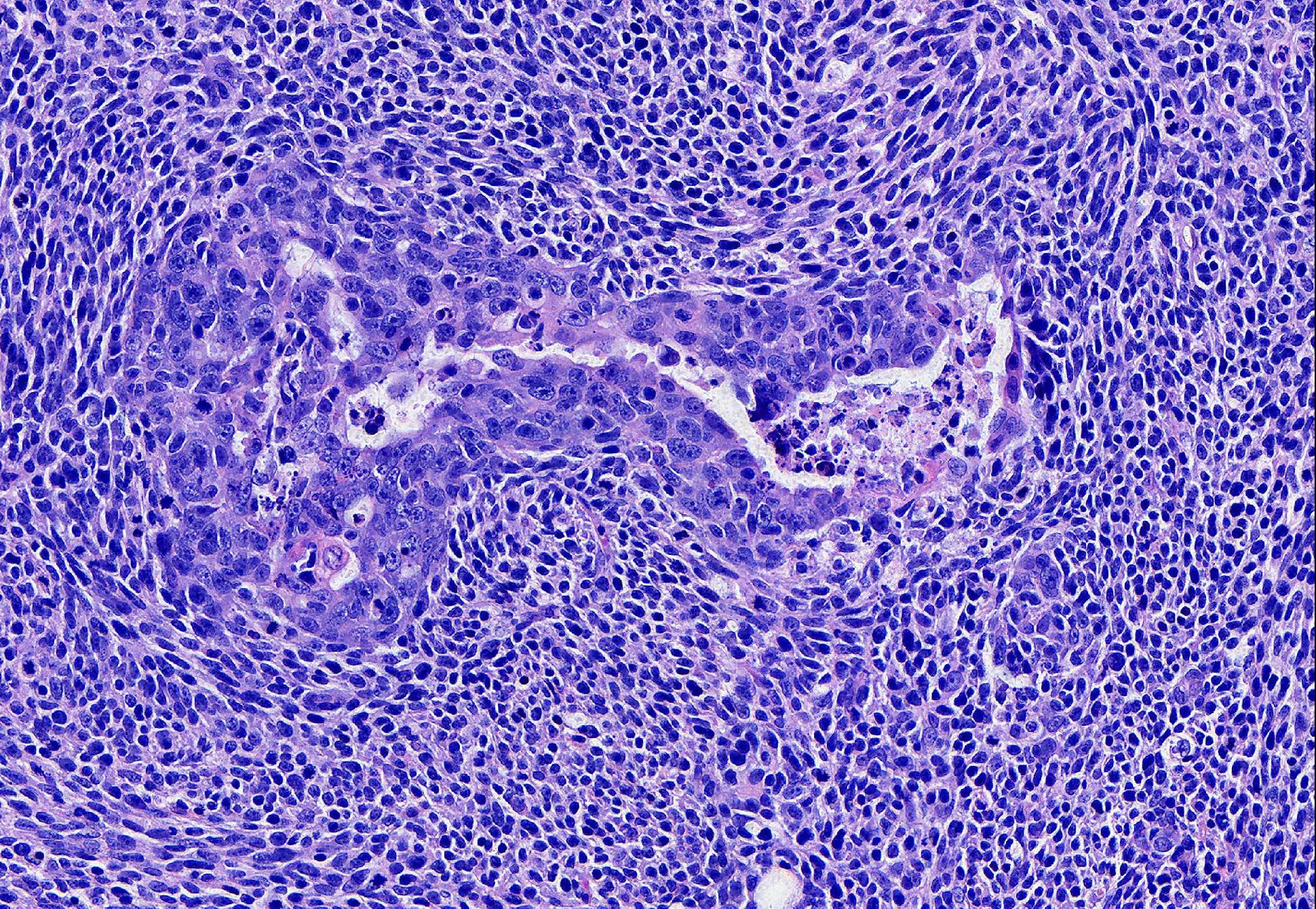
The epithelial population is composed of polygonal cells in irregular tubules and islands with frequent central necrosis. The cells have round to oval to irregular nuclei with large amounts of vesicular to coarsely stippled chromatin and a large central nucleolus. They have moderate amounts of eosinophilic cytoplasm. They exhibit marked anisocytosis and anisokaryosis with 29 mitotic figures in 10 HPF. Near the nipple, neoplastic epithelial cells are present without the neoplastic spindle population and are instead inciting a prominent scirrhous reaction. The neoplastic epithelial cells extend along the epithelium and fill the lumen of the teat sinus, where they are mixed with corpora amylacea.
The neoplastic spindle cell population typically surrounds the epithelial population and is composed of streams of spindle cells that have elongate nuclei with large amounts of coarse chromatin and a small nucleolus. They have small amounts of indistinct, eosinophilic cytoplasm. They exhibit moderate anisocytosis and anisokaryosis with 42 mitotic figures in 10 HPF. Narrow fingers of neoplastic spindle cells extend several millimeters from the main mass into the surrounding subcutaneous tissue.
The right superficial inguinal lymph node (not included) is extensively effaced by the neoplastic epithelial population and an associated scirrhous response.
Contributors Morphologic Diagnosis: Mammary gland: Carcinosarcoma.
Contributors Comment: Mammary neoplasia in cats occurs less commonly than in dogs but when it occurs, it is more likely to be malignant.4 The classification of mammary neoplasia is complex and neoplasms may be composed of elements of epithelial, basal/myoepithelial, or mesenchymal origin individually or in combinations. Subtyping of feline mammary neoplasia has been based on the classification scheme published in 1999 by the World Health Organization with some publications proposing updates that would bring the feline classification scheme closer to the recently updated guidelines for canine mammary neoplasia.4,5,9,17 Immunohistochemistry can be helpful (or critical) in some cases to help distinguish the cell types involved in a particular neoplasm. Cells of luminal epithelial origin are expected to express so-called luminal cytokeratins (CK7, CK8, CK18, CK19). Cells of basal or myoepithelial origin are expected to express so-called basal cytokeratins (CK5, CK6, CK14, CK17) as well as smooth muscle actin, vimentin, calponin, and p63.14
In this case, proliferative epithelial and spindle cell populations were identified. Both populations had high mitotic rates, atypia, and infiltrative growth to support malignancy. Furthermore, the epithelial population was found within sections of a draining lymph node. Based on the hematoxylin and eosin stained sections, the primary differentials considered were carcinosarcoma (malignant mixed mammary tumor), carcinoma and malignant myoepithelioma, or spindle cell carcinoma arising from tubular carcinoma. The cytokeratin cocktail (AE1/AE3) used for IHC in this case recognizes cytokeratins 1, 2, 3, 4, 5, 6, 7, 8, 10, 14, 15, 16, 19, so both luminal and basal/myoepithelial cells would be expected to stain. The lack of significant cytokeratin or smooth muscle actin expression in the spindle cell population made it unlikely that this population was of myoepithelial origin and would not be expected with a diagnosis of carcinoma and malignant myoepithelioma. The lack of cytokeratin expression in the vast majority of the spindle cells would also be unexpected in a spindle cell carcinoma.5 Given the presence of a distinct cytokeratin-expressing epithelial population and a distinct vimentin-expressing spindle cell population, a diagnosis of carcinosarcoma (malignant mixed mammary tumor) was made. Additional IHC for other markers of myoepithelium (calponin and p63) were not performed in our lab to further rule out a myoepithelial component.
Although feline mammary carcinosarcomas have appeared in several reports, they are rare and do not appear in more recent publications discussing prognostic evaluation of feline mammary neoplasia. 2,8,10,13,17 In dogs, carcinosarcomas of the mammary gland are also rare and appear to have an aggressive course with all dogs with carcinosarcoma in one prognostic study (8 cases) exhibiting metastasis and having a median survival of only 4.2 months.12 This particular cat had evidence of pulmonary (radiographic evidence) and lymph node (histologically confirmed) metastasis at the time of biopsy with progression of pulmonary nodules seen radiographically a month later. Mammary carcinosarcomas in dogs frequently have an osteosarcoma component,4,5 but this was not seen in any sections from this particular case.
In cats, tumors with malignant epithelial and mesenchymal components (variably referred to as carcinosarcomas, malignant mixed tumors, or sarcomatoid carcinoma) have been rarely described in other organs, including uterus11, salivary gland7, prostate16, lung3, digital apocrine glands6, pancreas15, and biliary system1.
JPC Diagnosis: Mammary gland: Carcinosarcoma, Domestic shorthair, feline.
Conference Comment: This case provided the rare opportunity to discuss the intricacies of feline mammary neoplasia, as well as the difficulties in precisely classifying the more rare variants.. Participants described a well-demarcated, multinodular neoplasm that expands the dermis composed of two types of cells: polygonal cells arranged in islands and trabeculae with central areas of comedonecrosis and spindle cells arranged in long interlacing streams separating islands of polygonal cells. Near the teat canal, there are areas of desmoplasia and large areas of necrosis with neoplastic cells present within the teat canal and lymphatic vessels.
Feline mammary tumors are much less common than their canine counterparts and are more often malignant. Consequently grading and staging are important in regards to prognosis. The Elston and Ellis grading system (used in women) has been adapted for cats as well as a more recent numeric grading system that bases tumor grade on the following criteria: lymphovascular invasion, nuclear form, and mitotic count. Prognostic factors include: epidemiologic factors (age, breed, reproductive status), clinical factors (staging, tumor size, lymph node invasion and metastasis, aggressiveness of surgery), and histological factors (type of tumor, grade).4
The conference moderator led a discussion on the published classification of feline mammary tumors. Hyperplasia and benign neoplasms are rare but are similar to whats found in bitches. Malignant mammary neoplasms are split into three groups: malignant epithelial neoplasms, malignant epithelial neoplasms special types, and malignant mesenchymal neoplasms sarcomas.
Table 1: Feline-specific malignant mammary gland tumors4
|
Malignant epithelial neoplasms |
|
|
Cribiform carcinoma |
Lumina lined by neoplastic cells that are very inconspicuous (compared to tubular carcinomas). |
|
Micropapillary invasive carcinoma |
In queens and males, more than 50% have a micropapillary pattern. |
|
Comedocarcinoma |
Multiple small areas of well-defined necrosis located at the center of nodules of neoplastic cells. Neutrophil infiltration is attributed to mastitis in addition to the neoplastic process. |
|
Anaplastic carcinoma |
Rare in cats. |
|
Intraductal papillary carcinoma |
Rare in cats, identical to what is seen in dogs. Malignant variant of intraductal papillary adenoma. |
|
Ductal carcinoma |
AKA feline complex carcinoma, malignant variant of the ductal adenoma which is composed of doubled layered neoplastic cells (luminal epithelial and basal cell components) forming cords, tubules, and solid areas around slit-like lumina. Ductal carcinomas are more solid with increased basal cells and less organized structure, more mitotically active with cellular atypia, and potentially areas of squamous differentiation and keratinization. |
|
Malignant epithelial neoplasms special types |
|
|
Adenosquamous carcinoma |
Areas of carcinoma (any type) and areas of squamous differentiation. |
|
Mucinous carcinoma |
Rare, contains an abundant mucoid matrix surrounding neoplastic epithelial cells. |
|
Lipid-rich carcinoma |
Rare in cats, identical to what is seen in dogs. |
|
Malignant mesenchymal neoplasms sarcomas |
|
|
Inflammatory carcinoma |
Clinical diagnosis characterized by sudden onset of subcutaneous edema, erythema, and pain in the ventral abdomen. The common histologic feature is large emboli of neoplastic cells in dermal lymphatics. |
The contributors morphologic diagnosis was discussed and debated at length. For expert evaluation, the case was sent to Dr. Michael Goldschmidt, Professor Emeritus at University of Pennsylvania, who commented that based on the morphology of the original HE slide (which was the only slide avaible to participants), a diagnosis of carcinosarcoma was perfectly acceptable. Several additional immunohistochemical stains were run to better categorize the neoplastic cell population. Both calponin and p63 were equivocal, staining some of the mesenchymal cell population but not all. Cytokeratin 5, 6, and 7 were negative for the mesenchymal cell population. Based on these results, we agree with the contributors diagnosis of carcinosarcoma.
Contributing Institution:
Cummings School of Veterinary Medicine
Tufts University
http://vet.tufts.edu/foster-hospital-small-animals/departments-and-services/pathology-service/
References:
- Cavicchioli L, Ferro S, Zappulli V, et al. Carcinosarcoma of the biliary system in a cat. J Vet Diag Invest. 2013; 25(5): 562-565.
- Fusaro L, Panarese S, Brunetti B, Sarli G, et al. Quantitative analysis of telomerase in feline mammary tissues. J Vet Diagn Invest. 2009; 21:369-373.
- Ghisleni G, Grieco V, Scanziani E, et al. Pulmonary carcinosarcoma in a cat. J Vet Diag Invest. 2003; 15: 170-173.
- Goldschmidt MH, Peña L, Zappulli. Tumors of the Mammary Gland. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: John Wiley & Sons, Inc; 2017: 723-765.
- Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117-131.
- Herraez P, Rodriguez F, Espinosa de los Monteros A, et al. Multiple primary digital apocrine sweat gland carcinosarcoma in a cat. Vet Rec. 2005; 157(12): 356-358.
- Kim H, Nakaichi M, Itamoto K, Taura Y. Malignant mixed tumor in the salivary gland of a cat. J Vet Sci. 2008; 9(3): 331-333.
- Mills SW, Musil KM, Davies JL, et al. Prognostic value of histologic grading for feline mammary carcinoma: a retrospective survival analysis. Vet Pathol. 2015;52:238-249.
- Misdorp W, Else RW, Hellmen E, et al. Histological classification of mammary tumors of the dog and the cat. In: World Heath Organization, ed. International Histological Classification of Tumors of Domestic Animals. Second ser. Vol 7. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology; 1999.
- Paniago JDG, Vieira ALS, Ocarino NM, Serakides R, et al. Mammary carcinosarcoma in cat: a case report. Arq Bras Med Vet Zootec. 2010; 62(4): 812-815.
- Papparella S, Roperto F. Spontaneous uterine tumors in 3 cats. Vet Pathol. 1984; 21:257-258.
- Rasotto R, Berlato D, Goldschmidt MH, and Zappulli V. Prognostic significance of canine mammary tumor histologic subtypes: an observational cohort study of 229 cases. Vet Pathol. 2017; doi: 10.1177/0300985817698208
- Soares M, Madeira S,Correia J, Ferreira F, et al. Molecular based subtyping of feline mammary carcinomas and clinicopathological characterization. The Breast. 2016; 44-51.
- Sorenmo KU, Rasotto R, Zappulli V, Goldschmidt MH. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet Pathol. 2011;48:85-97.
- Yamamoto R, Suzuki K, Mitsumor K, et al. Pancreatic carcinosarcoma in a cat. J Comp Path. 2012; 147: 223-226.
- Zambelli D, Cunto M, Bettini G, et al. Successful surgical treatment of a prostatic biphasic tumor (sarcomatoid carcinoma) in a cat. J Fel Med and Surg. 2010; 12: 161-165.
- Zappulli V, Rasotto R, Caliari D, et al. Prognostic evaluation of feline mammary carcinomas: a review of the literature. Vet Pathol. 2015;52:46-60.