Wednesday Slide Conference, 2025-2026, Conference 4, Case 4
Signalment:
5 year old castrated male Nubian goat (Capra aegagrus hircus).History:
The goat presented to Ohio State Large Animal Services with a 10 day history of lethargy, depression and difficulty standing. Previously he had a wet cough that resolved with Nuflor by the rDVM, though he continued to have mucopurulent discharge and a mild cough. Upon presentation, he was quiet, alert, responsive, and in sternal recumbency. Bloodwork suggested renal disease based on azotemia and metabolic acidosis (see laboratory results below), with an unremarkable urinary bladder and small hypoechoic region in the renal medulla on ultrasound. He received a blood transfusion and fluid therapy, but was not observed to urinate. He continued to decline clinically, and was humanely euthanized.Gross Pathology:
On gross examination, there was mild serosanguineous pericardial effusion, similar fluid within the entire length of the trachea as well as pulmonary edema. The urogenital tract was unremarkable.Laboratory Results:
PCV/TP:- Pre-transfusion = 11/ 7.4
- Post-transfusion = 17/ 6.4
NOVA Panel:
- pH = 7.467
- BUN = 303mg/dL
- Creatinine = 15.5mg/dL
- pCO2 = 31.4mmHg
- HCO3 = 22.9mmol/L
Profile:
- BUN = 380mg/dL
- Creatinine = 17.8mg/dL
- Phosphorous = 4.5mg/dL
- Calcium (total) = 7.7mg/dL
- Sodium = 138meq/dL
- Potassium = 3.73meq/dL
- Chloride = 86.7meq/dL
- Anion Gap = 39meq/dL
- Total protein = 5.8g/dL
- Albumin = 3.1g/dL
- Globulin = 2.7g/dL
Hemogram/ CBC:
- Plasma protein = 7.8g/dL
- HCT = 15%
- Hemoglobin = 3.8g/dL
- RBC = 7.3x10^12/L
- MCHC = 25.7g/dL
- Retic absolute = 32.1x10^9/L
- Total leukocytes = 19.9x10^9/L
- Seg neutrophils = 16.9x10^9/L
- Lymphocytes = 3.0x10^9/L
Parasitology (McMaster’s Fecal Float):
- Few strongyles and occasional Eimeria were detected in post-mortem colonic contents. No parasites were detectable in the posts-mortem abomasal contents.
Microscopic Description:
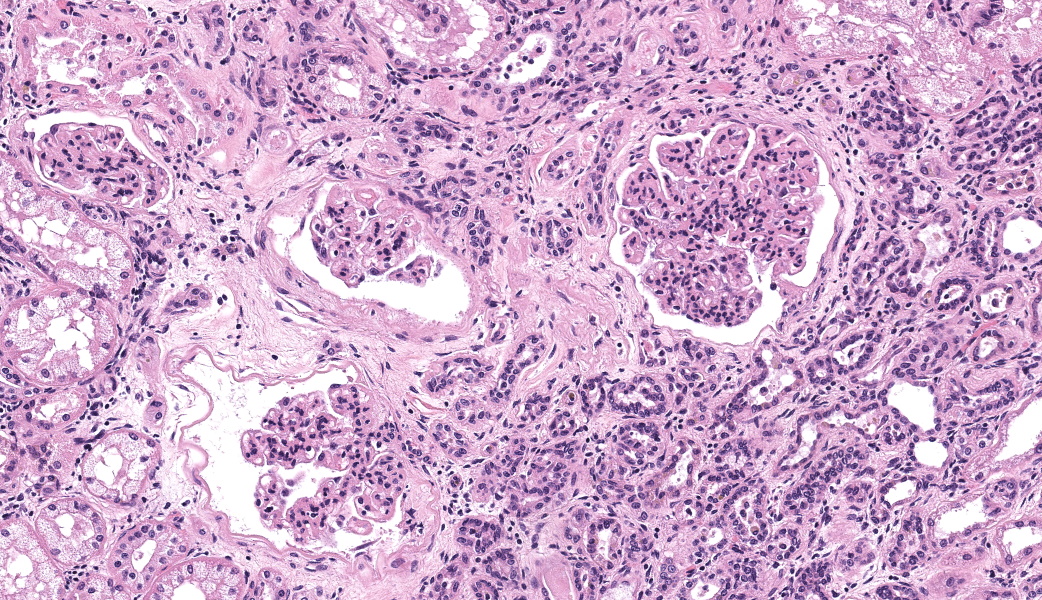
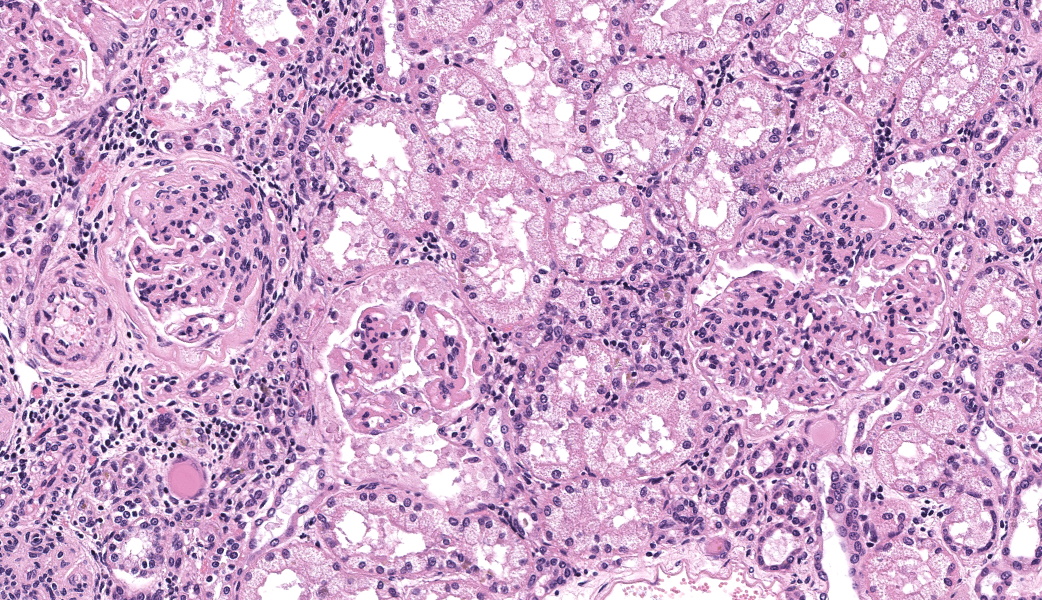
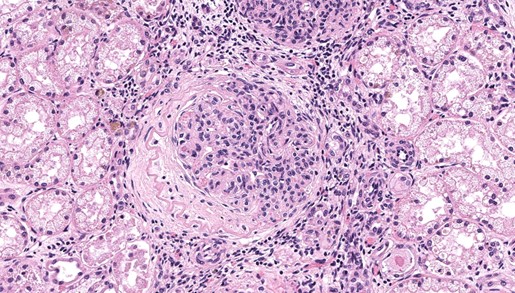
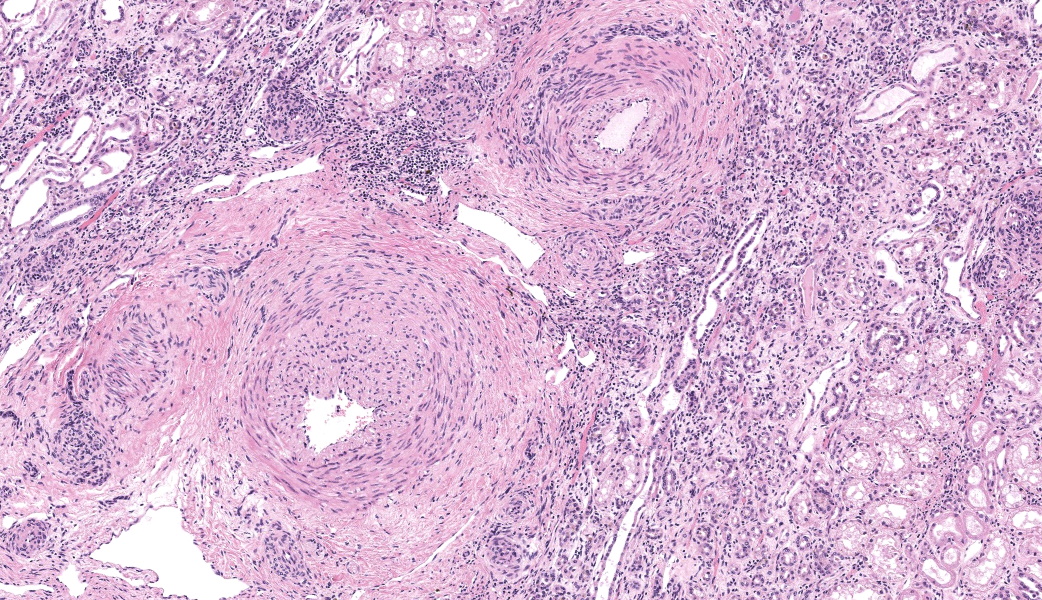
There are multiple sections of kidney that include cortex, medulla, and papilla. All of the glomeruli are affected to varying degrees of severity. The glomeruli are globally hypercellular, within both the endocapillary and mesangial compartments. Capillary walls are markedly thickened by eosinophilic matrix. There is frequent dilation of Bowman’s capsules and segmental collapsed glomerular tufts/ capillaries with expansion of mesangium with eosinophilic matrix (segmental sclerosis). Less than 25% of the glomeruli are globally sclerotic. Occasionally glomerular tufts are segmentally to globally effaced by eosinophilic mesangial matrix, and mesangial and intracapillary dark brown (hemosiderin) and yellow (hematoidin) pigment with scattered hemosiderin laden macrophages within Bowman’s space. There is infrequent segmental loss of podocytes with proliferation of eosinophilic matrix and cells that adhere the glomerular tuft to Bowman’s capsule (crescents). Several glomerular capillary loops are filled with fibrin thrombi.Over half of the tubules are characterized by attenuated epithelium, sloughed hypereosinophilic necrotic intraluminal epithelium, and/or piling of basophilic tubular epithelium (regeneration). Often tubules are dilated with eosinophilic proteinaceous material (hyaline casts), and contain intraluminal basophilic refractile material (mineralization). Infrequently, tubules are filled with degenerate neutrophils. There is a mild loss of tubules with expansion of the interstitium by fibrous matrix and aggregates of lymphocytes with fewer plasma cells. There are multiple arcuate arteries with an expanded tunica intima by increased numbers of smooth muscle cells and fibrous matrix, and/or thickened and hypercellular tunica medias (arteriosclerosis).
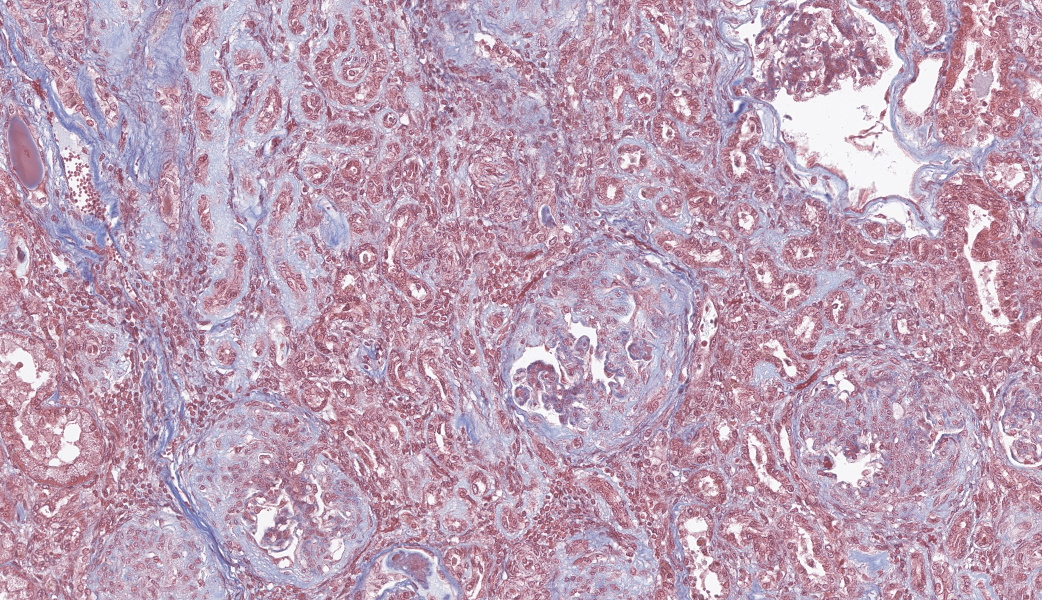
Special stains included Masson’s Trichrome, Periodic-Acid Schiff (PAS), and Jones Methenamine Silver (JMS). The PAS demonstrated that the thickened mesangium and glomerular capillary loops were expanded by PAS positive material that often-formed double contours, spike-like projections and encircled bright magenta deposits using the Trichrome stain. Both the PAS and Trichrome highlight the collapsed capillary loops of the globally sclerotic glomeruli with expansion of Trichrome and PAS positive matrix.
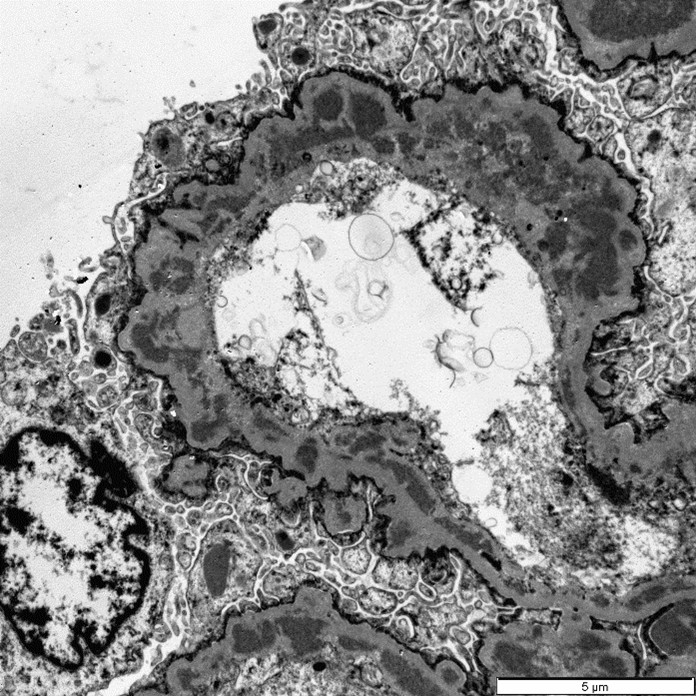
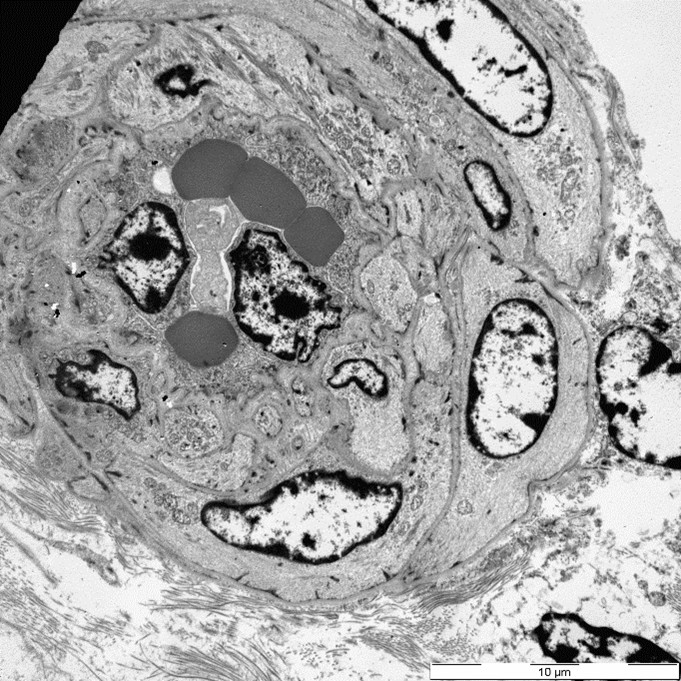
Three glomeruli were available for ultrastructural evaluation by transmission electron microscopy (TEM). One glomerulus had collapsed capillary loops and fibrillary material in Bowman’s space. All glomeruli had numerous large electron dense deposits within the capillary walls in intramembranous, subendothelial, mesangial, and subepithelial locations. Subepithelial deposits were associated with spike-like projections and circulations of glomerular basement membrane material. One glomerulus is segmentally sclerotic with loss of podocytes and denuded capillary walls. The afferent/ efferent arterioles have severely hypertrophied endothelial cells with markedly narrow lumens. In one of the arterioles the circulating erythrocytes are distorted.
Contributor's Morphologic Diagnoses:
Kidney: Diffuse global to segmental marked chronic active immune complex mediated membranoproliferative glomerulonephritis with superimposed thrombotic microangiopathy, tubular necrosis and atrophy, interstitial fibrosis and lymphoplasmacytic interstitial nephritisContributor's Comment:
Immune complex (IC) glomerulonephritis (GN) is classified as membranous, proliferative, mesangioproliferative, and membranoproliferative. Membranous glomerulonephritis is characterized by a remodeled basement membrane secondary to IC deposition on the abluminal surface of the glomerular basement membrane leading to normocellularity to mild hypercellularity. Proliferative glomerulonephritis histologically has increased cellularity without alterations to the glomerular basement membranes and may be associated with IC deposition. Mesangioproliferative glomerulonephritis histologically has increased cellularity within the mesangium with IC deposition usually between the endothelium and the glomerular basement membrane. Membranoproliferative glomerulonephritis (MPGN) is characterized by endocapillary and mesangial cell proliferation with remodeling of the capillary loop due to IC deposition usually between the endothelium and the glomerular basement membrane4. In the present case, the immune complexes were present in multiple locations hence the use of “mixed MPGN”.ICGN results from circulating antigen-antibody complexes of non-glomerular origin that localize in the glomerulus and are able to be detected via immunofluorescence or TEM as electron-dense deposits. The immune complexes often contain complement, as well. IC deposition is thought to occur when concentrations of antigen and antibody are equal, or when antigens are in slight excess in comparison to antibody concentrations.4 Another hypothesis is that some antigens, such as those derived from viruses like feline leukemia virus, bacteria, and parasites, such as Dirofilaria.4,2 Experimentally chronic antigenemia leading to ICGN is modeled by repeatedly injecting small doses of endogenous or exogenous antigens. Although caprine arthritis and encephalitis virus (CAEV) has not been previously reported to cause ICGN in goats, the herd of the present case was positive for CAEV and is thought to be the source of consistent antigen stimulation. There was a study on naturally infected sheep with Maedi-visna virus and associated glomerular lesions; however, there was lack of TEM and glomerular staining (PAS, JMS, and Trichrome) to fully characterize the lesions of ICGN.1
Thrombotic microangiopathy is characterized by endothelial cell damage that potentially produces thrombocytopenia and hyaline thrombi.3,5 Causes include genetic factors, malignant hypertension, pregnancy, drugs, transplantation, and infections.3 TMA is the lesion in Greyhounds with idiopathic cutaneous and renal glomerular vasculopathy, as well as the well-characterized hemolytic uremic syndrome in humans associated with Shiga or Shiga-like exotoxin of Escherichia coli O157:H7.3,4 In humans there are also several viruses associated with and thought to cause TMA.3,5 Therefore, in the present case, the superimposed lesion of TMA is likely related to CAEV infection and/or hypertension. The acute tubular necrosis in this case is likely related to ischemia possibly related to the vasculopathy as indicated by the arcuate arteriosclerosis and TMA lesions.
Contributing Institution:
The Ohio State University, Department of Veterinary Biosciences, Columbus Oh. www.vet.osu.eduJPC Diagnoses:
Kidney: Glomerulonephritis, membranoproliferative, chronic, diffuse, severe, with glomerulosclerosis, lymphoplasmacytic interstitial nephritis, tubular degeneration, necrosis, and regeneration, and arteriolar fibrointimal hyperplasia and sclerosis.JPC Comment:
Closing out our fourth conference, this case graced participants with an opportunity to see a lovely correlation between light microscopy and electron microscopy (EM) findings. It’s not often that EM images are sent in as part of Wednesday Slide Conference submissions, but it’s always a treat when they are! Many thanks to the contributor for providing both a wonderful slide and beautiful EM images. Together, they truly did paint the whole picture for this EM-worthy condition.Because the EM images were provided, a review of the ultrastructural anatomy of a glomerular capillary profile and its surrounding structures ensued, with focus on the primary and secondary foot processes of the visible podocyte and the different layers of the glomerulus. However, the main show was the electron-dense, granular, irregularly shaped clumps of immune complexes (IC) within subepithelial, intramembranous, and subendothelial spaces of the glomerular tuft. Having IC deposition demonstrably in all three of those locations within the glomerulus assisted with the determination of the “mixed” type of glomerulonephritis in this case. Conference participants favored “membranoproliferative” based on the H&E slide, but acknowledged that this case did not fit 100% into that box upon review of the EM images and accepted the use of the term “mixed” in discussion. Typing of glomerulonephritis is more difficult to do with light microscopy alone due to some histologic similarities between the types, bringing home the point that, for definitive characterization of glomerulonephritis, EM is indispensable.
There was discourse on differentiating membranoproliferative from membranous glomerulonephritis based on histologic features, as some conference participants went one way or the other in their morphologic diagnoses. To summarize, membranoproliferative glomerulonephritis will have an increased number of nuclei within the glomerular tuft, mesangial cell proliferation, and often, the presence of recruited neutrophils. Membranous glomerulonephritis typically just has an increased amount (thickening) of basement membranes. One conference participant inquired on the histologic differences between synechiae and crescents in the glomerulus. Synechiae were described as the touching of the visceral layer of Bowman’s capsule to the parietal layer without fibrosis, whereas crescents have a fibrous component and demonstrate full adhesion of the glomerulus to the capsule. Conference participants did not think there was enough histologic evidence of thrombotic microangiopathy include it in the morphologic diagnosis in this particular case.
So, the renal glomerulus…what a champion. The glomerulus is a physiologic heavy lifter made up of three distinct layers: fenestrated endothelium, basement membrane, and podocytes. These layers enable the glomerulus to fulfill its destiny as a highly selective filter of blood based on compound size and charge, ridding the body of wastes while retaining essential materials. Glomerular filtrate is passed into the surrounding Bowman’s capsule before moving into the proximal convoluted tubules to continue its journey. For all its hard work and contributions to the bodily society, glomeruli are relatively fragile and prone to injury. Immune-complex glomerulonephritis occurs when the concentration of antigen-antibody complexes within the body becomes too high for the body to clear in a timely fashion. The size, charge, and insolubility of ICs, along with excessive complement activation secondary to their deposition, can overwhelm the body's clearance mechanisms and result in deposition into the glomerulus.
Complexes are deposited into glomerular capillaries due to the basement membrane's negative charge. The basement membrane will attract positively charged pre-formed complexes, but can also form IC complexes on itself if a positively charged antigen binds to the glomerulus directly and is then tagged by an antibody.5 The ICs trigger an immune response by either calling in inflammatory cells via their sheer presence or by activating resident glomerular cells to release cytokines, vasoactive substances, and prothrombotic compounds. Either way, this ultimately damages the endothelium, epithelium, and mesangium of the affected glomerulus, resulting in glomerulonephritis.5
The primary mediator of IC glomerulonephritis is the complement system, specifically the membrane attack complex (MAC), which is made up of complement components C5b complexed with C6-9 to create C5b6789. The MAC generally does not achieve sufficient quantities to result in cell lysis in this IC glomerulonephritis, but inserts into the membranes of glomerular cells and causes their conversion into resident inflammatory effector cells that then damage the glomerulus.5 Couple this process with the lymphocytes and plasma cells that show up in response, the poor affected glomerulus is set on a course of irreversible damage. Mesangial cells and capillary endothelial cells try their best to combat the glomerular destruction by proliferating and remodeling, hence the “proliferative” part of this diagnosis, but this can only do so much if the antigen-antibody complexes continue to circulate in high numbers and continuously deposit into glomeruli.
All in all, this case was easily the most heavily discussed both during conference and the morphologic diagnosis session, where there was highly spirited discussion between staff on the most appropriate morphologic diagnosis. The attending residents were able to hear a variety of opinions on these lesions and, ultimately, consensus (more or less) was reached. Many thanks again to this contributor for a truly high-value learning case!
References:
- Angelopoulou K, Brellou GD, Vlemmas I. Detection of maedi-visna virus in the kidneys of naturally infected sheep. Journal of comparative pathology. 2006;134: 329-335.
- Long JD, Rutledge SM, Sise ME. Autoimmune Kidney Diseases Associated with Chronic Viral Infections. Rheumatic diseases clinics of North America. 2018;44: 675-698.
- Lopes da Silva R. Viral-associated thrombotic microangiopathies. Hematology/oncology and stem cell therapy. 2011;4: 51-59.
- Maxie MG. Jubb, Kennedy, and Palmer's pathology of domestic animals, Sixth ed., vol. 2. St. Louis (MO): Elsevier; 2016.
- Nangaku M, Couser WG. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin Exp Nephrol. 2005;9(3):183-91.
- Salter T, Burton H, Douthwaite S, Newsholme W, Horsfield C, Hilton R. Immune Complex Mediated Glomerulonephritis with Acute Thrombotic Microangiopathy following Newly Detected Hepatitis B Virus Infection in a Kidney Transplant Recipient. Case reports in transplantation. 2016;2016:
- Sementilli A, Moura LA, Franco MF. The role of electron microscopy for the diagnosis of glomerulopathies. Sao Paulo Med J. 2004;122(3):104-9.