Wednesday Slide Conference, Conference 13, Case 3
Signalment:
Juvenile, female, giant Pacific octopus (Enteroctopus dofleini)
History:
This giant Pacific octopus arrived at the submitting zoo after an extended period at an outgoing airport. During the first week at the zoo, the animal was eating well but was staying at the bottom of the enclosure. Shortly thereafter, the octopus stopped eating and was found to have several injuries consistent with self-mutilation and damage by the cohabitant anemones. Water quality was within normal limits. The octopus was found deceased a few days later.
Gross Pathology:
Macroscopic lesions included skin erosions and ulcerations and distal limb amputations. The digestive gland was uniformly soft, tan-pink, and bulging on cut section. There were no significant macroscopic lesions of the renal appendages.
Laboratory Results:
Aerobic culture of the digestive gland yielded Carnobacterium sp. (1+ mixed) and Vibrio sp. (4+ predominant).
Microscopic Description:
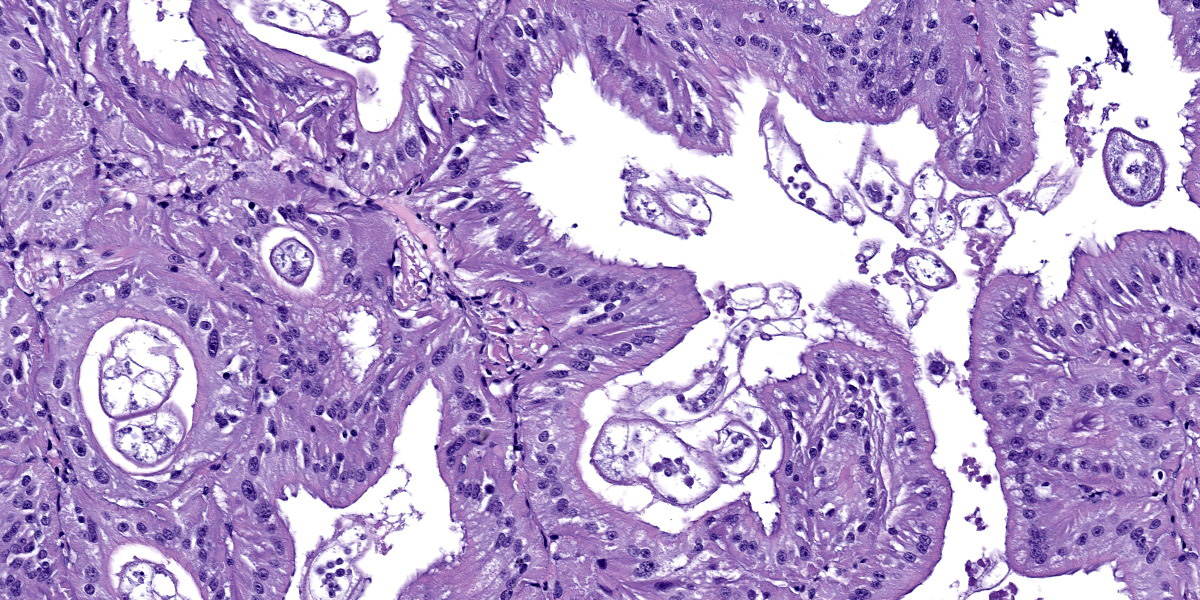
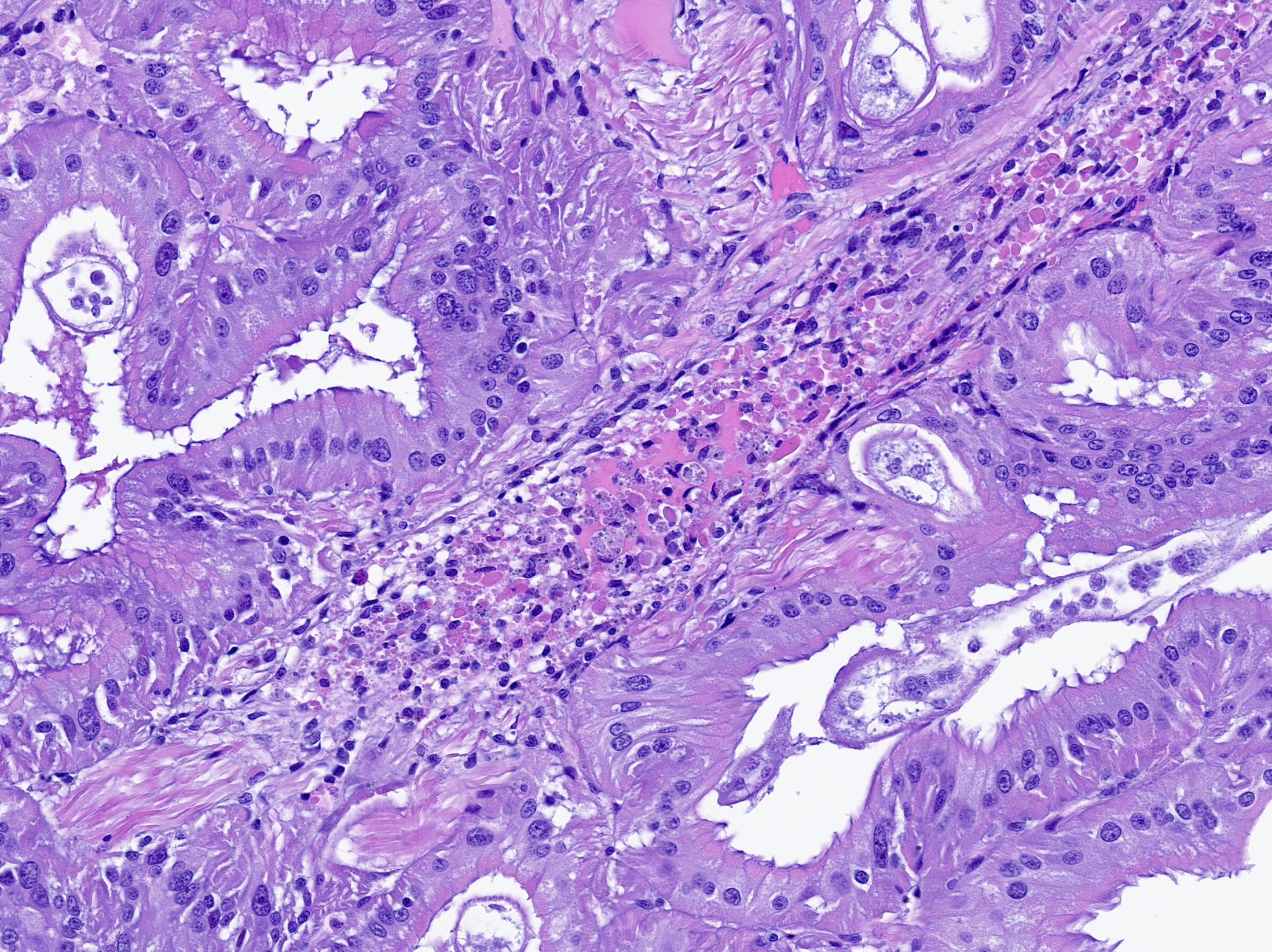
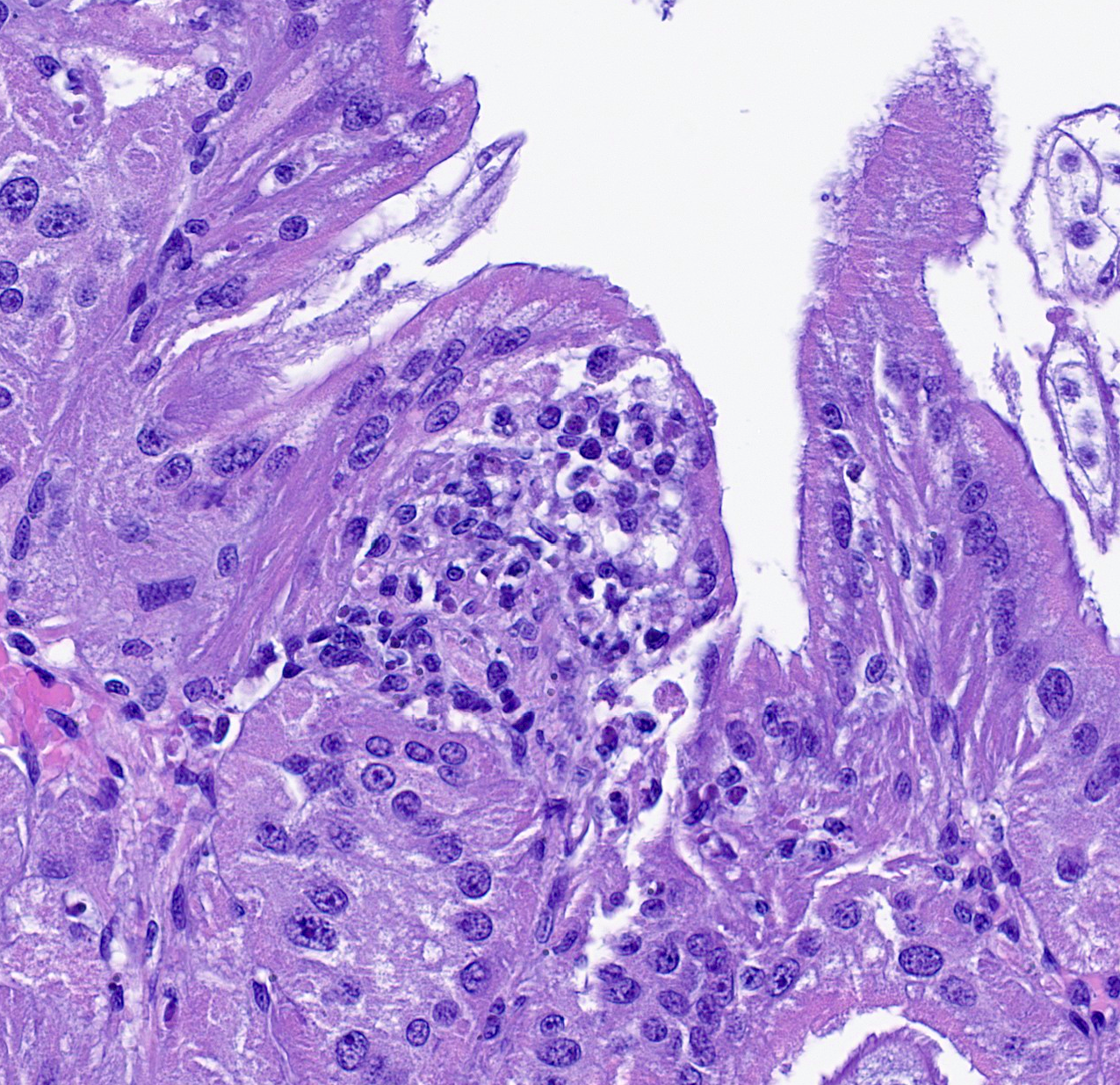
Renal appendage: The renal appendage is composed of folds and tubules lined by simple columnar epithelium supported by a fibrovascular stroma and separated by venous sinuses. The epithelial cells are large, with basal nuclei, mild apical vacuolation, and a brush border. Anisocytosis and anisokaryosis of the renal epithelial cells is moderate to marked. Copious globular, eosinophilic material is present within tubule lumina, and homogeneous, eosinophilic secretory product multifocally fills sinus lumina. Along the epithelial surfaces, there are innumerable cross-sectional and tangential profiles of multicellular, vermiform organisms at various stages of maturation, with no apparent associated inflammatory reaction. The organisms measure approximately 25 to 100 micrometers in diameter and consist of a central axial cell enveloped completely by a layer of ciliated peripheral cells. Several large peripheral cells with short or inapparent cilia comprise the anterior ends of the organisms (interpreted as the calotte), which commonly abut the renal epithelial cells and obscure the brush border. At these sites, the epithelial cells are variably attenuated. Within the axial cell cytoplasm in a majority of the organisms, there are one or more developing embryos and single cells (agametes or fertilized eggs). The developing embryos are multicellular, often organized into an elongate structure or forming a round to oval cluster of cells (vermiform and infusoriform embryos).
Contributor’s Morphologic Diagnosis:
Renal appendage: intraluminal dicyemid organisms, widespread, marked, chronic
Contributor’s Comment:
The renal organs of octopuses consist of folded tissue that protrudes into the renal sac. The degree of tissue folding generally increases with the size of the organism and increases excretory capacity.10 The folds form tubules lined by a simple columnar epithelium and separated by sinuses that originate from the vena cava. Within the tubule and renal sac lumina, one may find a variety of worm-like dicyemid organisms that are often attached to the renal epithelium without eliciting an inflammatory response.1,3
Dicyemids (phylum Dicyemida) are mesozoans found within the renal organs of many cephalopod species, and were an incidental finding in this giant Pacific octopus (Enter octopus dofleini). Dicyemids have been variably described as endosymbionts and as endoparasites, as they attach to the renal epithelium of the host, absorb nutrients from the urine through their peripheral cells, and can be present in large numbers. Regardless, they have not been shown to damage the renal organs or be detrimental to the host.1 Their postulated benefits include acidification of the urine to aid excretion of ammonia and maintenance of urine flow by their ciliary movements.1,10,14
There are 149 documented dicyemid species to date, all of which exhibit a remarkably simple body plan that left many questions about their evolutionary origins.15 They were initially classified as mesozoa for presumptively representing an intermediate between protozoa and metazoa. While the phylogenetic particulars of these organisms are arguably uncertain, current evidence indicates they arose from metazoan ancestors as a result of evolutionary simplification with genome reduction.4,14
The dicyemid life cycle consists of vermiform and infusoriform stages. Mature dicyemids are vermiform and exist within the renal organ as a nematogen, a primary rhombogen, or a secondary rhombogen.11 These adults possess a central axial cell with intracytoplasmic agametes that is fully surrounded by 8 to 30 ciliated peripheral cells. Four to ten peripheral cells at the anterior aspect of the organism form a calotte, which interacts with the host renal epithelium.15
Nematogens reproduce asexually. An agamete within the axial cell cytoplasm develops into a vermiform embryo, which then exits the adult through a gap between peripheral cell membranes or by penetrating a peripheral cell.12,13 The vermiform larva matures into either another nematogen or a primary rhombogen and remains within the host. In contrast, primary rhombogens and secondary rhombogens, which transition from nematogens rather than mature from vermiform larvae, reproduce sexually. An agamete within the axial cell develops into an infusorigen, a hermaphroditic gonad that gives rise to oocytes and spermatocytes. Fertilization results in development of infusoriform embryos, which escape the parent organism in a manner similar to vermiform embryos.10 While the vermiform stages are confined to the renal organs, infusoriform larvae can exit the renal organ, escape the original host, and eventually infect a new host by an unknown mechanism.12 Many dicyemid species exhibit high host specificity, and two or three species are commonly found within a single cephalopod host.15
Vermiform larvae exhibit a body plan similar to the adults. Contrarily, infusoriform larvae are composed of a higher number and greater variety of cells, resulting in diverse body organizations of at least 14 different cell types.8 The number of peripheral cells in the adults, the shape and organization of the calotte, the number of total and peripheral cells in vermiform embryos, and the number and organization of cells in infusoriform embryos are species-specific.7-9,12 Distinguishing between dicyemid species, rhombogen and nematogen forms, and vermiform and infusoriform embryos in routine histologic sections seems quite challenging, and may be best attempted using tissue smears and particular fixation and storage techniques.
Contributing Institution:
University of Minnesota Veterinary Diagnostic Laboratory
https://vdl.umn.edu/
JPC Diagnosis:
Renal appendage: Nephritis, hemocytic, acute, multifocal, mild with vasculitis.
JPC Comment:
We thank the contributor for sharing this unique tissue with us in conference. Invertebrate pathology was a recent focus of Veterinary Pathology2 and the present section is an opportunity to build familiarity both with invertebrate anatomy and a common background commensal organism in octopuses. Additionally, tissue (digestive gland) from this case was also presented at the 2024 Davis-Thompson Foundation Invertebrate Histology Seminar – Dr. Elise Ladouceur was one of the moderators and weighed in similarly on this case.
The contributor provides a terrific summary of dicyemid background biology and intersection with normal octopus physiology. Although not a lesion per se, recognizing these as normal organisms and avoid diagnosing pathologic parasitism merits discussion among a wider audience. Conference participants hotly debated how to best capture the role of dicyemids in this case. As we typically do not create morphologic diagnoses for non-lesions, we were rescued in this case by a less prominent pathologic finding. Nonetheless, we concur with the presence of renal dicyemids, a routine finding in octopuses that should not be interpreted as a lesion. Dr. Ladouceur also noted that reduced numbers or absence of renal dicyemids may represent loss of fitness, though this remains speculative.
Although somewhat subtle, there was also evidence of multifocal inflammation in the renal appendage. This was likely secondary to bacterial sepsis, consistent with the contributor’s aerobic culture of the digestive gland which yielded both Carnobacterium and Vibrio species. Hemocytic inflammation effaced the walls of hemolymphatic vessels and was associated with karyorrhexis, consistent with vasculitis. Although not part of the reviewed tissues, follow up with the contributor confirmed that this animal had severe interstitial inflammation in the digestive gland centered on gram negative, curved bacilli which was consistent with Vibrio spp. infection. Vibrio spp. have been frequently isolated from skin lesions and/or organs or fluid (hemolymph) of larval, juvenile, and adult octopuses.5,6 Additionally, other bacteria associated with mortality and sepsis in octopuses include Pseudomonas spp. and Aeromonas spp.,5,6 though discerning the ability of these bacteria to cause primary disease, especially in mixed bacterial culture with Vibrio, remains uncertain. Preventative health measures such as tank cleaning and water quality management remain important considerations for captive cephalopod husbandry.6
References:
- Anadón R. Functional Histology: The Tissues of Common Coleoid Cephalopods. In: Gestal C, Pascual S, Guerra Á, Fiorito G, Vieites JM, eds. Handbook of Pathogens and Diseases in Cephalods. Springer Open; 2019.
- Dennis MM, LaDouceur EEB. Special focus issue on invertebrate pathology: A growing discipline requiring veterinary diagnosticians. Veterinary Pathology. 2023;60(5):501-502.
- Dill-Okubo JA, Berzins IK, LaDouceur EEB, Camus AC. Mollusca: Cephalopoda. In: LaDouceur EEB, ed. Invertebrate Histology. John Wiley & Sons, Inc.; 2021.
- Drábková M, et al. Different phylogenomic methods support monophyly of enigmatic ‘Mesozoa’ (Dicyemida + Orthonectida, Lophotrochozoa). Proc R Soc B. 2022;289(1978):20220683.
- Farto R, Armada SP, Montes M, Guisande JA, Pérez MJ, Nieto TP. Vibrio lentus associated with diseased wild octopus (Octopus vulgaris). J Invertebr Pathol. 2003 Jun;83(2):149-56.
- Farto R, Fichi G, Gestal C, Pascual S, Nieto TP. Bacteria-Affecting Cephalopods. In: Gestal C, Pascual S, Guerra Á, Fiorito G, Vieites J, eds. Handbook of Pathogens and Diseases in Cephalopods. Springer, Cham. 2019. https://doi.org/10.1007/978-3-030-11330-8_8
- Furuya H, Hochberg FG, Tsuneki K. Calotte morphology in the phylum Dicyemida: niche separation and convergence. J Zool. 2003;259:361-373.
- Furuya H, Hochberg FG, Tsuneki K. Cell Number and Cellular Composition in Infusoriform Larvae of Dicyemid Mesozoans (Phylum Dicyemida). Zool Sci. 2004;21:877-889.
- Furuya H, Hochberg FG, Tsuneki K. Cell number and cellular composition in vermiform larvae of dicyemid mesozoans. J Zool. 2007;272:284-298.
- Furuya H, Ota M, Kimura R, Tsuneki K. Renal Organs of Cephalopods: A Habitat for Dicyemids and Chromidinids. J Morphol. 2004;262:629-643.
- Furuya H. Progenesis in dicyemids. Invertebr Biol. 2024;142:e12419.
- Furuya H, Tsuneki K. Biology of Dicyemid Mesozoans. Zool Sci. 2003;20:519-532.
- Hisayama N, Furuya H. Escape Processes in Embryos of Dicyemids (Phylum Dicyemida). J Parasitol. 2023;109(5):496-505.
- Lu TM, Kanda M, Furuya H, Satoh N. Dicyemid Mesozoans: A Unique Parasitic Lifestyle and a Reduced Genome. Genome Biol Evol. 2019;11(8):2232-2243.
- Nakajima H, Fukui A, Suzuki K, Tirta RYK, Furuya H. Host Switching in Dicyemids (Phylum Dicyemida). J Parasitol. 2024;110(2):159-169.