Wednesday Slide Conference, Conference 14, Case 4
Signalment:
Six-month-old Brangus, bovine (Bos taurus taurus)
History:
In the period between December 2018 and February 2019, a farm owner recorded the death of five six-month-old calves, including nursing and weaned calves, from a group of 117. Cattle were vaccinated against foot-and-mouth disease, rabies, and clostridiosis, and the herd was kept in native pastures. Affected calves presented similar clinical signs that included fever, apathy, severe respiratory distress, nasal discharge, and diarrhea. Clinical course lasted approximately three days, and calves died spontaneously. All calves that presented clinical signs were treated with enrofloxacin, dipyrone, imizole, and levamisole; however, no significant clinical improvement was observed. One of the deceased calves was submitted for postmortem examination.
Gross Pathology:
The calf showed good body condition. Gross findings included mild jaundice in the subcutaneous tissue, oral mucosa, and ocular conjunctiva. At the opening of the thoracic cavity, the lungs were diffusely pink-red, non-collapsed, with a rubbery texture, and presented interlobular edema on the cut surface. In the cranioventral areas of the lung and in the ventral regions of the caudal lobes, multifocal red-tan and firm areas (2-7 cm in diameter) were observed. At the inspection of the abdominal cavity, the liver was markedly enlarged, with round edges, and diffuse orange discoloration. The gallbladder was distended and filled with grumous bile. The spleen was also severely enlarged. No alterations were observed in other organs.
Laboratory Results:
Culture and isolation of Salmonella spp. were attempted using fresh samples of lung. These samples were added in buffered peptone water and incubated overnight (37°C). Following the pre-enrichment, they were inoculated in selective enrichment broth (Rappaport-Vassiliadis broth) and incubated at 42°C overnight. Bacterial isolates were submitted for serotyping using a slide micro-agglutination test. Fresh lung samples were tested through RT-PCR for bovine respiratory complex agents (BoHV/PI-3/BRSV) and Influenza D. Lung sections were submitted for immunohistochemistry (IHC) using a commercial polyclonal antibody against Salmonella spp. (Biogenesis®), as previously described by JUFFO et al. (2017).4
Salmonella spp. was isolated from the lungs, and the serotype identified was Salmonella Dublin. Mild, multifocal,cytoplasmic labeling (IHC) of the bacteria was detected in lung macrophages and freely in the pulmonary interstitial space. RT-PCR for bovine respiratory complex agents (BoHV/PI-3/BRSV) and Influenza D were negative.
Microscopic Description:
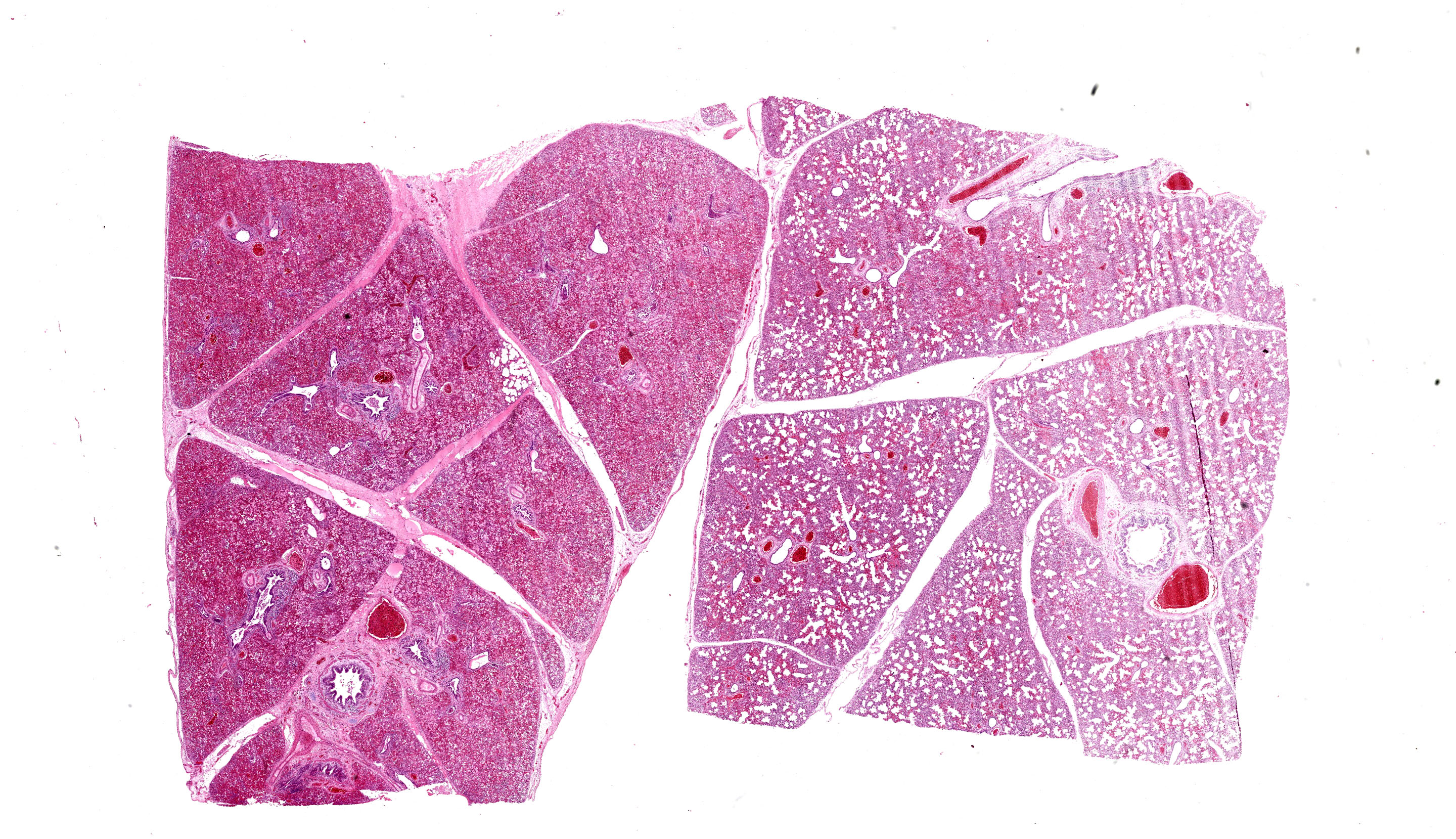
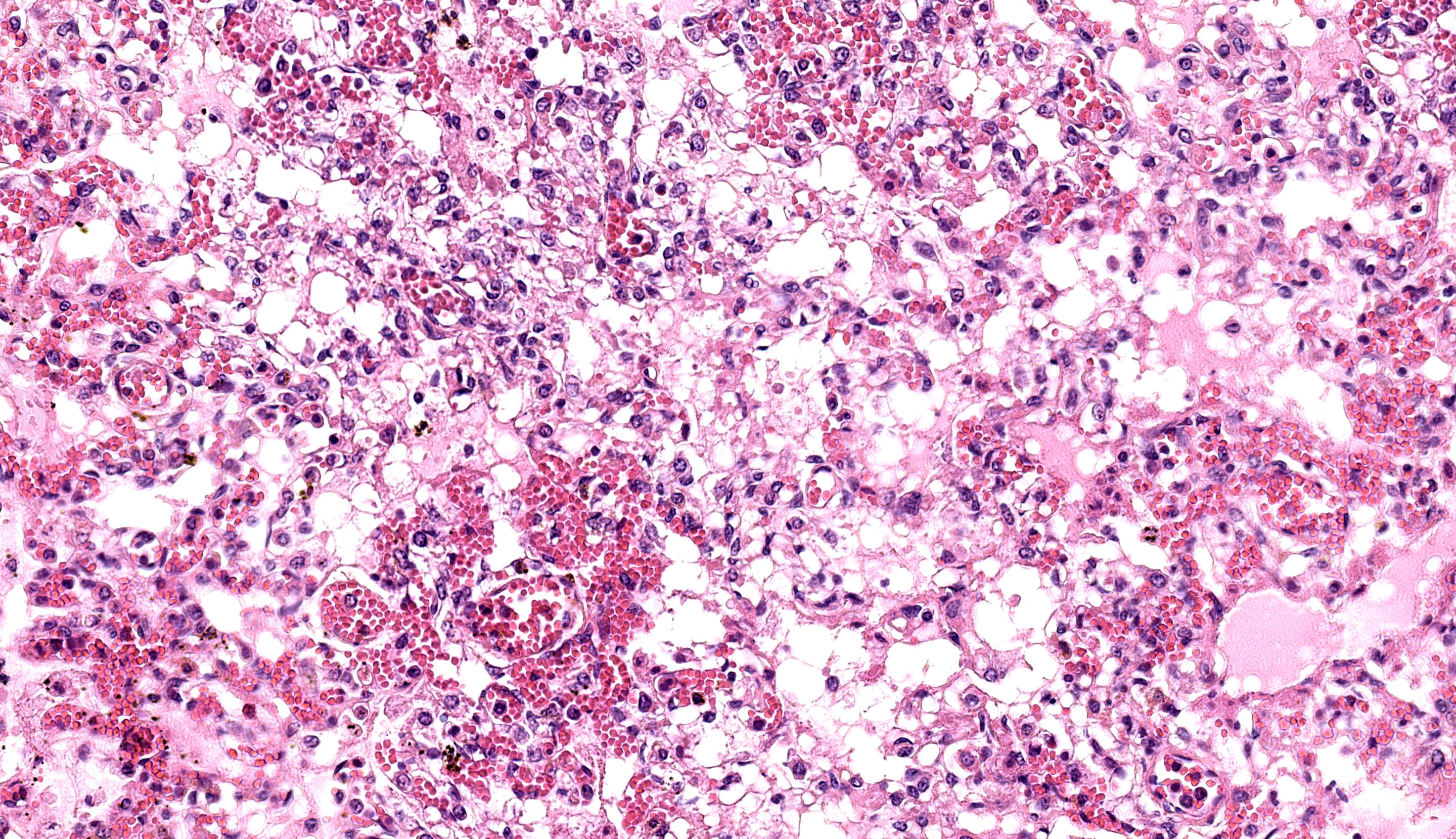
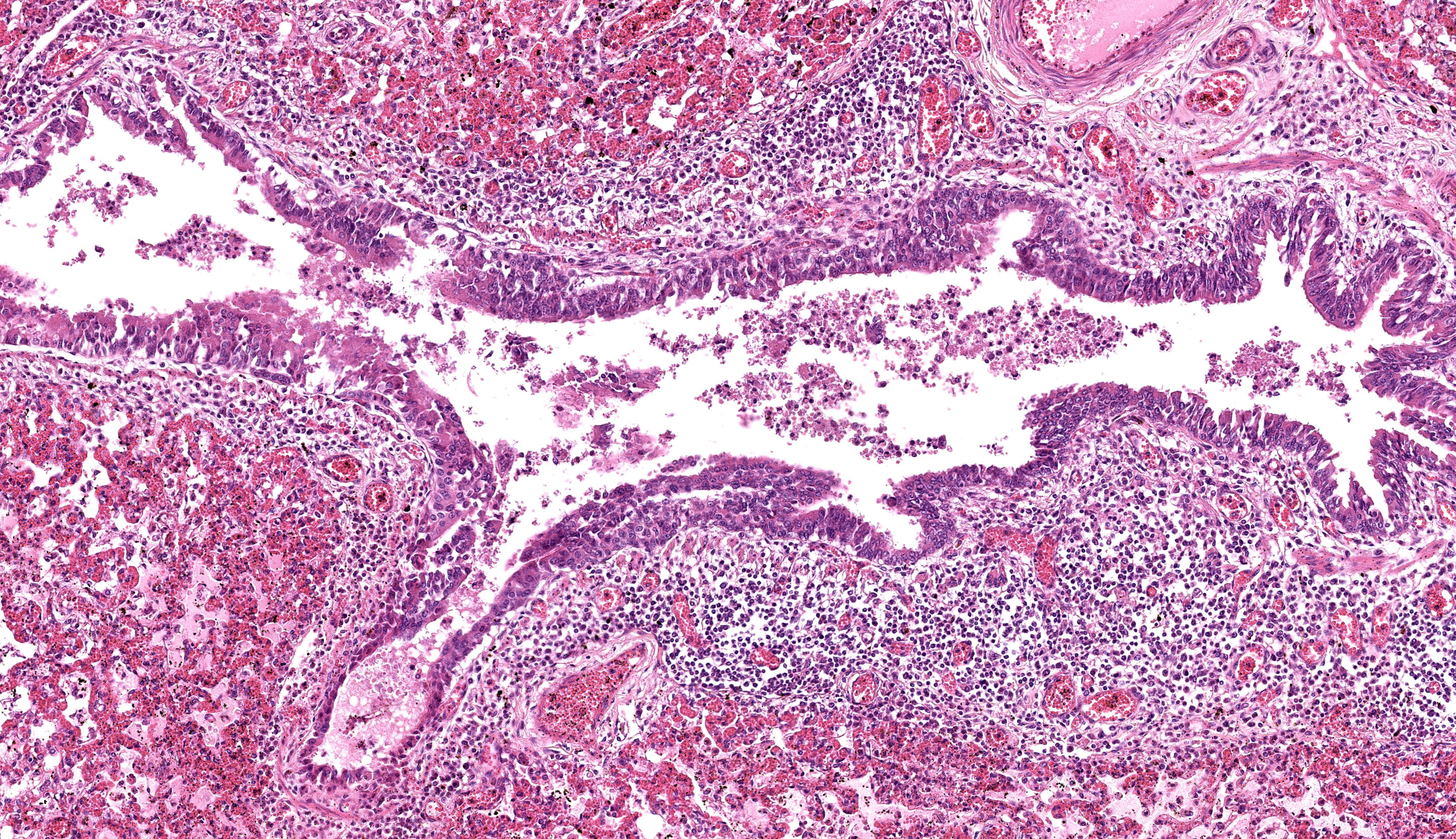
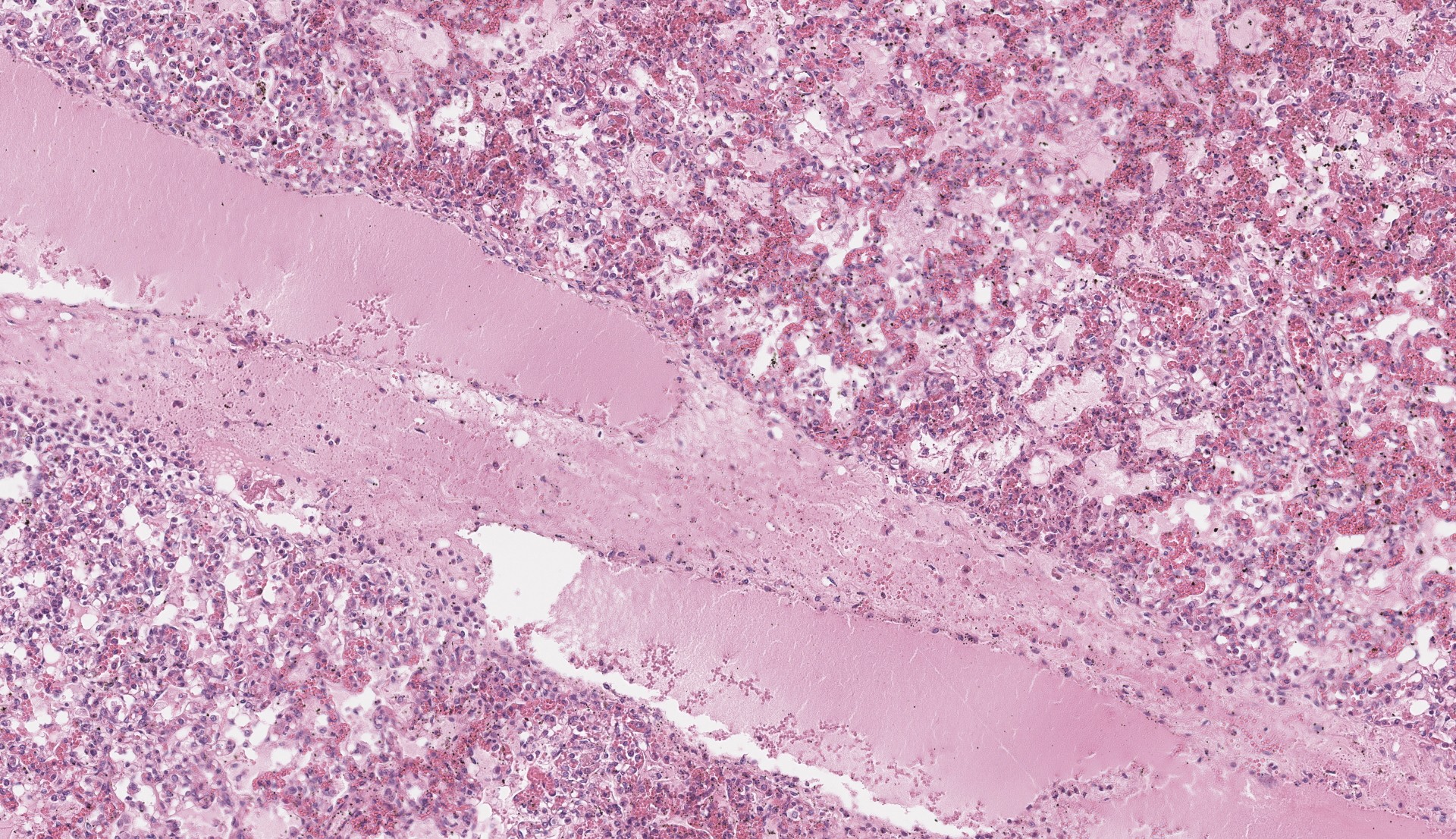
Each submitted slide presents a section of lung. Diffusely, alveolar septa are markedly thickened and expanded by severe inflammatory infiltrate of macrophages, lymphocytes, and neutrophils, as well as moderate to marked type II pneumocyte hyperplasia. Similar inflammatory infiltrate composed of large foamy macrophages, lymphocytes, neutrophils, and rare multinucleated syncytial cells, is observed filling alveolar spaces, and less frequently the lumen of bronchioles and bronchi. Multifocal, moderate areas of fibrin deposition, intra-alveolar hemorrhage and mild necrosis are also observed. Additionally, multifocal areas of mild thrombosis, and marked expansion of interlobular septa by eosinophilic homogenous material (edema) are observed.
Contributor’s Morphologic Diagnosis:
Lung: Pneumonia, interstitial, lymphohistiocytic, and neutrophilic, multifocal to coalescing, severe, with type II pneumocyte hyperplasia, and interlobular edema, Brangus, bovine.
Contributor’s Comment:
Pathological, immunohistochemical and microbiological findings supported the diagnosis of Salmonella-associated pneumonia in this case. In cattle, salmonellosis is predominantly caused by Salmonella enterica subsp. enterica serovar Typhimurium and S. enterica subsp. enterica serovar Dublin.1,9,11 Salmonella Typhimurium may cause septicemia and is commonly detected in outbreaks of enteric disease in calves. In contrast, S. Dublin, a host adapted serovar in cattle, is predominantly associated with septicemia across all age groups in cattle.11 Salmonellosis in cattle is often characterized by watery or mucoid diarrhea containing fibrin and blood, septicemia, respiratory disease, weight loss, and abortions.8,1 Lesions include enterocolitis, pneumonia, paratyphoid nodules in the liver, necrotic foci in the kidney, and splenomegaly.2
In cases of Salmonella-associated pneumonia, the most representative changes include interstitial pneumonia, and therefore, the main differential diagnosis should include other important causes of bronchointerstitial pneumonia in calves, such as BoHV (bovine herpesvirus), PI-3 (parainfluenza virus) and BRSV (bovine respiratory syncytial virus), which were ruled out by molecular assays in this case. An outbreak of septicemic salmonellosis with lung involvement has been described in the North region of Brazil associated with serovar Dublin.5 Salmonellosis with primary lung involvement, in the absence of enteric lesions, has been reported in the Central-West region of Brazil,3 and in Southern Brazil, by our research group.7 Likewise, a study conducted in the United States of America reported similar lung lesions in young cattle.9
The fecal-oral route represents the main route of transmission, and disease development is associated with bacterial efficiency in invading the intestinal mucosa, colonizing lymphoid tissues, and evading host immune response. Affected animals or asymptomatic carriers may spread the disease in the herd.8 Young calves (< 6 months of age) are more vulnerable to the disease and may be infected a few hours after calving; nonetheless, adult cattle may also develop the clinical disease.6, 8 Potential predisposing factors for the development of clinical salmonellosis include concomitant diseases, stressors and immunosuppression, which may favor the development of clinical disease or may prompt cattle to become asymptomatic carriers.8 Infection in cattle may also be favoured by age and physiological stage.8 In this case, we believe that weaning-associated stressors may have contributed to the development of clinical salmonellosis.
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
http://www.ufrgs.br/patologia
JPC Diagnosis:
Lung: Pneumonia, interstitial, lymphohistiocytic and neutrophilic, subacute, multifocal to coalescing, severe, with type II pneumocyte hyperplasia, thrombosis, and edema.
JPC Comment:
The final case of this conference is also a pneumonia which allows for a thoughtful comparison with Case 3. Dr. Brown emphasized several subtle aspects that hint at the underlying pathogenesis. There is hypoplasia of bronchus-associated lymphoid tissue (BALT) as well as a large thrombus in section which are suggestive of a septicemia.
The expansion of the alveolar septa is partially lymphohistiocytic like the previous case, though the distribution is very different. In the present case, alveolar septal expansion is diffuse whereas in the previous case, inflammation was multifocal and more prominent at the bronchoalveolar junction. This case fits better with a hematogenous portal of entry whereas Case 3 is more consistent with an aerogenous agent.
The conference concluded with a brief review of the role of bacteria in pneumonias. In bacterial bronchopneumonia, aerogenous entry of the agent and direct infection of the airway leads to exudation of the alveoli and bronchioles, largely with neutrophils and originating at the bronchiolar-alveolar junction. Conversely, bacterial interstitial pneumonias are the result of indirect action through cytokine effects and endotoxemia. Pulmonary intravascular macrophages (PIMs) have received increased attention for their role in a variety of diseases.12 In particular, these macrophages secrete proinflammatory cytokines (e.g. TNF- α) in response to both intracellular and extracellular pathogens and or related markers (e.g. LPS). These cytokines subsequently contribute to capillary dysfunction through a variety of changes such as disruption of endothelial tight junctions.12 In concert with the action of LPS on endothelial cells (i.e. activation), the large degree of edema and fibrin in this case is hardly surprising. Macrophage activation (MAS) has also been identified as a contributor to COVID-19 related lung pathology,13 though delineating whether cytokine-induced changes are local to the lung or systemic vasculature has spawned a related term of MAS-like immunopathology. In classic MAS, macrophages across tissue lines (e.g. Kupffer cells and alveolar macrophages) are activated concurrently, leading to hemophagocytosis and an eventual consumption coagulopathy with secondary liver dysfunction.13 In COVID-19 however, hemophagocytosis is localized as part of a response to diffuse alveolar damage.13 Common shared outcomes underscore similarities in molecular mechanisms of disease, particularly in the role of macrophage activation and cytokine production.
References:
- Carrique-Mas JJ, Willmington JA, Papadopoulou C, Watson EN, Davies RH. Salmonella infection in cattle in Great Britain, 2003 to 2008. Vet Rec 2010(167):560- 565.
- Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: JF ZACHARY, Pathologic Basis of Veterinary Disease, 2018, 6.ed. SaintLouis, Missouri: Elsevier, 324-411.
- Guizelini CC, Pupin RC, Leal CRB, Ramos CAN, Pavarini SP, Gomes DC, Martins TB, Lemos RAA. Salmonellosis in calves without intestinal lesions. Pesq Vet Bras 2019; 39(8):580-586.
- Juffo GD, et al. Equine salmonellosis in southern Brazil. Trop Anim Heal and Prod 2017; 49: 475–482.
- Marques ALA, Simões SVD, Garino Jr F, Maia LA, Silva TR, Riet-Correa B, Lima EF, Franklin Riet-Correa F. Salmonellosis outbreak by serovar Dublin in calves in Maranhão. Pesq Vet Bras 2013; 33(8):983-988.
- Mohler VL, Izzo MM. Salmonella in calves. Vet Clin North Am Food Anim Pract 2009; 25:37-54.
- Molossi FA, Cecco BS de, Henker LC, Vargas TP, Lorenzett MP, Bianchi MV, Lorenzo C, Sonne L, Driemeier D, Pavarini SP. Epidemiological and pathological aspects of salmonellosis in cattle in southern Brazil. Cienc Rural 2021; 51(3): e20200459.
- Nielsen LR. Salmonella Dublin in dairy cattle: use of diagnostic tests for investigation of risk factors and infection dynamics. 219 p. PHD thesis - Department of Animal Science and Animal Health, Royal Veterinary and Agricultural University, Denmark, 2003.
- Pecoraro HL, Thompson B, Duhamel GE. Histopathology case definition of naturally acquired Salmonella enterica serovar Dublin infection in young Holstein cattle in the northeastern United States. J Vet Diagn Invest 2017; 29(6):860–864.
- Peek SF, Hartmann FA, Thomas CB, Nordlund KV. Isolation of Salmonella spp from the environment of dairies without any history of clinical salmonellosis. Vet Med Sci 2004; 225(4):574- 577.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: M. G Maxie. Pathology of Domestic Animals, Jubb, Kennedy & Palmer´s. 2016, 6.ed. St. Louis, Missouri: Elsevier, 2, 117-176.
- Sun Z, Chen X, Liu J, Du Y, Duan C, Xiao S, Zhou Y, Fang L. PRRSV-induced inflammation in pulmonary intravascular macrophages (PIMs) and pulmonary alveolar macrophages (PAMs) contributes to endothelial barrier function injury. Vet Microbiol. 2023 Jun;281:109730.
- McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 Jul;2(7):e437-e445.