Signalment:
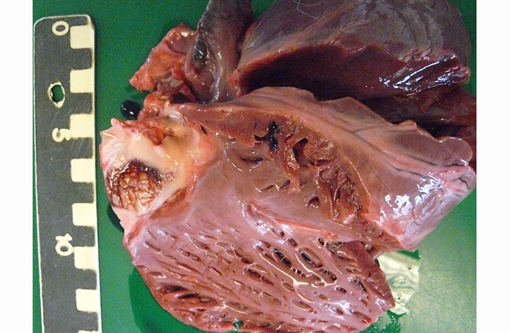
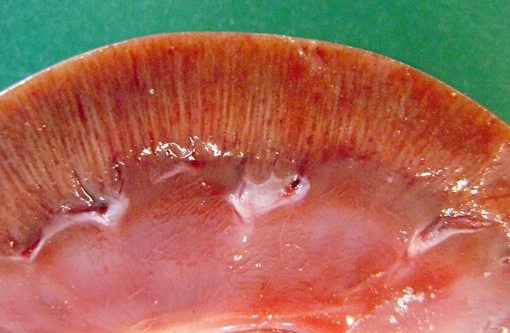
Gross Description:
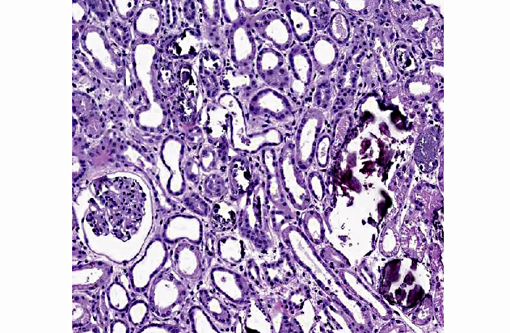
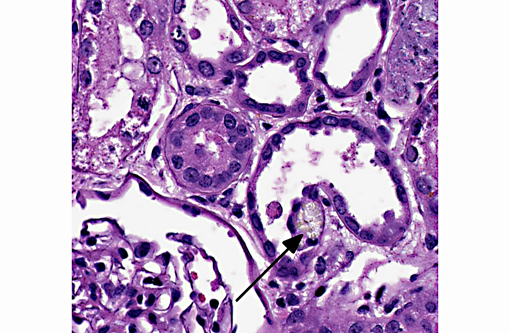
Histopathologic Description:
Morphologic Diagnosis:
1. Kidney: Tubular degeneration, necrosis and loss with intratubular calcium deposits and calcium oxalate crystals.
2. Kidney: Nephritis, interstitial, lymphoplasmacytic, multifocal, mild.
Lab Results:
In serum collected on the day of presentation at the clinic ethylene glycol or glycolic acid was not detected. Fresh renal tissue collected at the time of necropsy showed presence of glycolic acid.
Condition:
Contributor Comment:
EG poisoning commonly occurs in dogs and cats after accidental ingestion of antifreeze solution. Cats are more susceptible than dogs, but dogs are more commonly affected.(3) EG is readily absorbed from the intestinal tract, but is in itself of low toxicity. While most EG is eliminated in the urine, some is metabolized by alcohol dehydrogenase to glycoaldehyde and its metabolites glycolic acid (GA), glyoxylate and oxalate. EG is rapidly metabolized while GA accumulates in plasma, and is detectable for a longer time period.(2) Glycoaldehyde and glyoxylate have been considered to be the primary nephrotoxic metabolites.(3) However, recent studies show that the calcium oxalate crystals might be most important for the renal toxicity.(4)
EG toxicity may progress through three stages. The first stage of EG toxicity is characterized by central nervous depression. The second stage is characterized by metabolic acidosis and is seen 12-24 hours after ingestion, and GA is the major contributor to this. The third stage includes oxalic acid excretion, nephropathy and eventual renal failure.(2,3)
Renal failure is due to toxic tubular necrosis and a renal edema that compromises the intrarenal blood flow. Tubular changes are most severe in proximal tubules and range from hydropic degeneration to necrosis to regeneration. The characteristic calcium oxalate crystals may be found in tubular lumina, in tubular cells and in the interstitium. While few calcium oxalate crystals may be seen in chronic tubular obstruction, large numbers of these crystals in renal tubules are virtually pathognomonic for EG poisoning.(3)
JPC Diagnosis:
Conference Comment:
Acid-base homeostasis is tightly regulated by major buffers such as hemoglobin and the bicarbonate buffer system as well as by minor buffers that include inorganic phosphate and plasma proteins.(5) The bicarbonate buffer system plays a major role in acid-base regulation and acts through the equilibrium reaction H2O + CO2â H2CO3âH+ +HCO3- to control the amount of hydrogen ions in (and thus the pH of) the blood.(1,5) The ratio of HCO3-/ H2CO3 determines the blood pH: An excess of acid (acidosis) leads to a decrease in blood pH (acidemia); whereas an excess of base (alkalosis) causes an increase in blood pH (alkalemia).(1) Disturbances in acid-base status are classified as either metabolic or respiratory, based on the underlying mechanism. Metabolic acidosis, the most common acid-base disturbance, is due to the production of acid by a pathologic metabolic processes.(5) Decreased plasma HCO3- (or serum TCO2 concentrations, which is another way of measuring HCO3-) indicate metabolic acidosis.(1) In most species, plasma HCO3- or serum TCO2 concentrations of 15 to 20 mmol/L is interpreted as moderate metabolic acidosis; whereas in the dog and cat, 12 to 17 mmol/L is consistent with moderate metabolic acidosis. Severe metabolic acidosis occurs when plasma HCO3- or serum TCO2 concentrations are less than 15 mmol/L in most species and less than 12 mmol/L in the dog and cat.(1)
Common causes of metabolic acidosis include the following(1,5):
- loss of bicarbonate (such as occurs with severe diarrhea, or decreased synthesis and loss of NaHCO3 by the renal tubules)
- excess in organic acids (titration acidosis)
- lactic acidosis (from anaerobic glycolysis in hypoxia and shock; or excessive bacterial catabolism of carbohydrates)
- ketoacidosis (acetoacetic acid and beta-hydroxybutyric acid in diabetic ketoacidosis, starvation or ketosis of ruminants)
- renal failure (uremic acids)
- acid toxicities (e.g. ethylene glycol toxicity)
As in this case, metabolic acidosis associated with EG toxicity is due to the EG metabolite, glycolic acid. Hyperkalemia, also as observed in this case, results from acidosis, as an excess of hydrogen ions causes a shift of potassium ions from intracellular to extracellular space. Additionally, oliguria in the acute renal failure stage prevents excretion of potassium by the kidneys.(1)
In addition to these findings, EG toxicity is also often accompanied by a high anion gap, due to the presence of the salt of the organic acid.1 Although not reported in this case, hypocalcemia is often observed in cases of EG toxicity; although renal disease can cause hypocalcaemia by a variety of mechanisms, in EG toxicosis, it is likely due to the sequestration of calcium in the formation of calcium oxalate crystals.(1)
References:
2. Hess R, Bartels MJ, Pottenger LH. Ethylene glycol: an estimate of tolerable levels of exposure based on a review of animal and human data. Arch Toxicol. 2004;78:671-680.
3. Maxie M, Newman S. Urinary system. In:Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol. 2. Edinburg, Scotland: Saunders Elsevier; 2007:425-522.
4. McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol. 2009;47:859-869.
5. Weiser G. Laboratory of acid-base disorders. In: Thrall MA, Weiser G, Allison RW, Campbell TW, eds. Veterinary Hematology and Clinical Chemistry. Ames, Iowa: Wiley-Blackwell; 2012: Kindle edition, location 17216 of 37098.Â