CASE 1: 1 (4152313-00)
Signalment:
6-month-old male (intact) mastiff (Canis familiaris).
History:
The patient was referred to a veterinary medical teaching hospital following an acute, 1-day-history of lethargy and inappetence that quickly progressed to obtundation. Bloodwork performed at the rDVM revealed too high to read ALT, elevated ALP (805 U/L), hypoalbuminemia (2.7 g/dL) and hypoglycemia (23 mg/dL). PT and aPTT were both prolonged at >100 sec and >300 sec, respectively. Protein and bilirubin were detected on a urine dipstick. A SNAP test for Leptosperosis was negative. Despite aggressive palliative therapy, the patient continued to decline and euthanasia was elected.
Gross pathology:
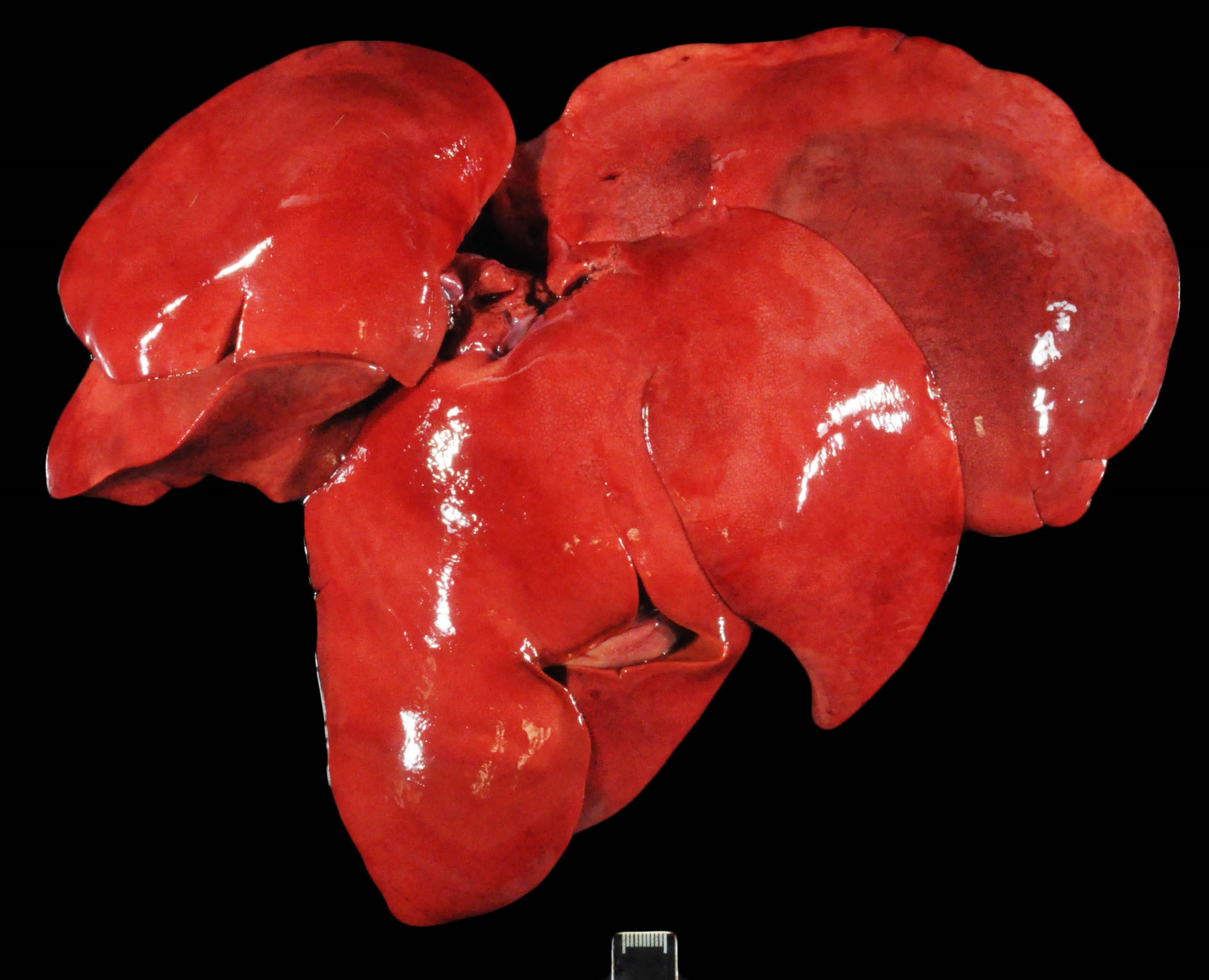
Only gross lesions pertinent to the histologic findings and case discussion are provided. The entire length of the gastrointestinal tract contained a small amount of intralumenal, watery, dark red to black ingesta and fecal material (melena). The intestinal mucosa was mottled pale pink/red/dark purple. The hepatic parenchyma was diffusely friable and mottled pale/bright red with a prominent reticular pattern (Image). The renal cortex was diffusely dull tan/purple. The medullary parenchyma was disrupted by dozens of bright red to dull purple lines that radiated from the level of the renal crest through the medulla.
Laboratory results:
Amanita (Liver): Trace detected
Aflatoxin (Liver): None detected
Microcystins (Stomach contents): None detected
Microscopic description:
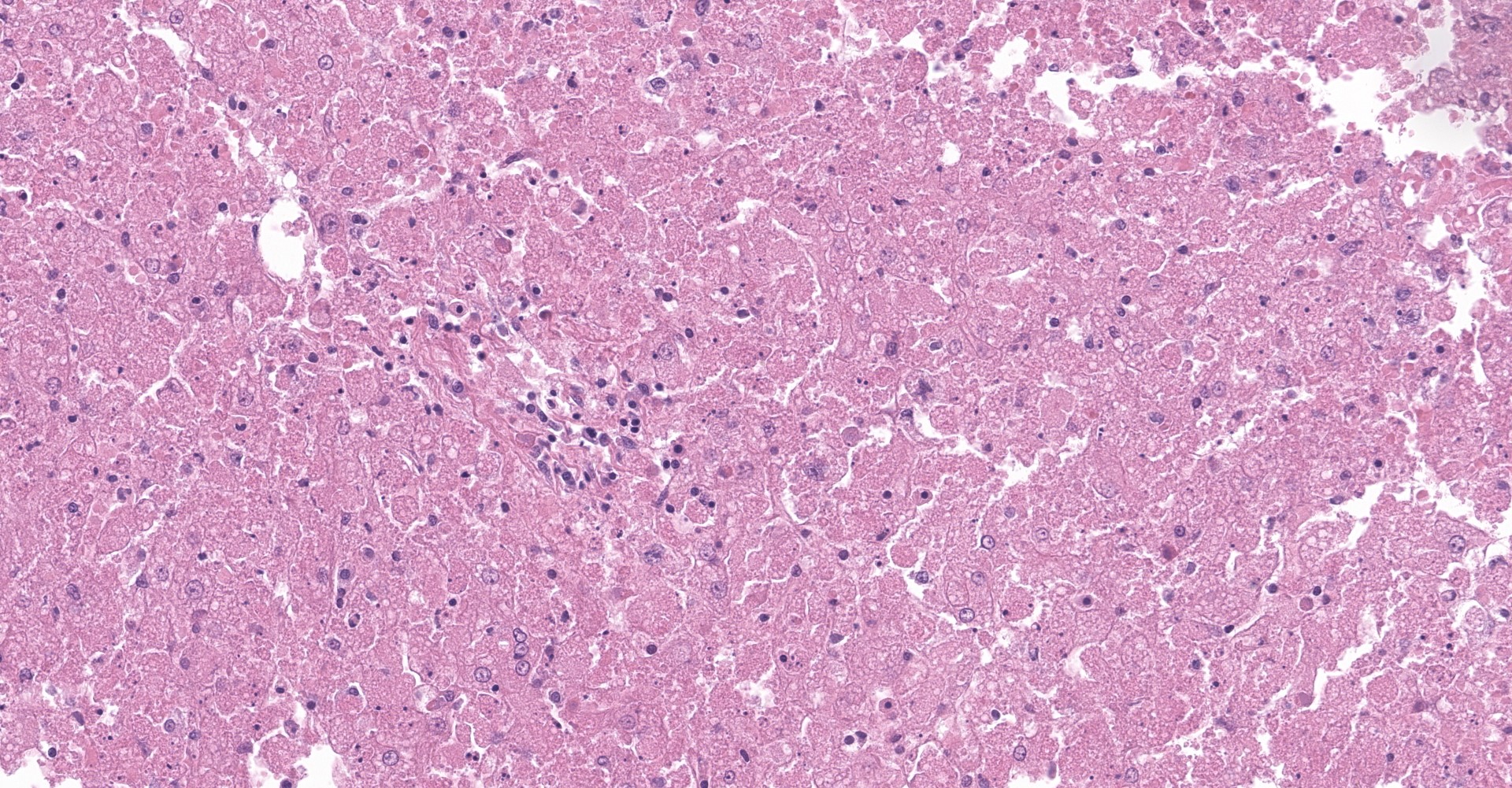
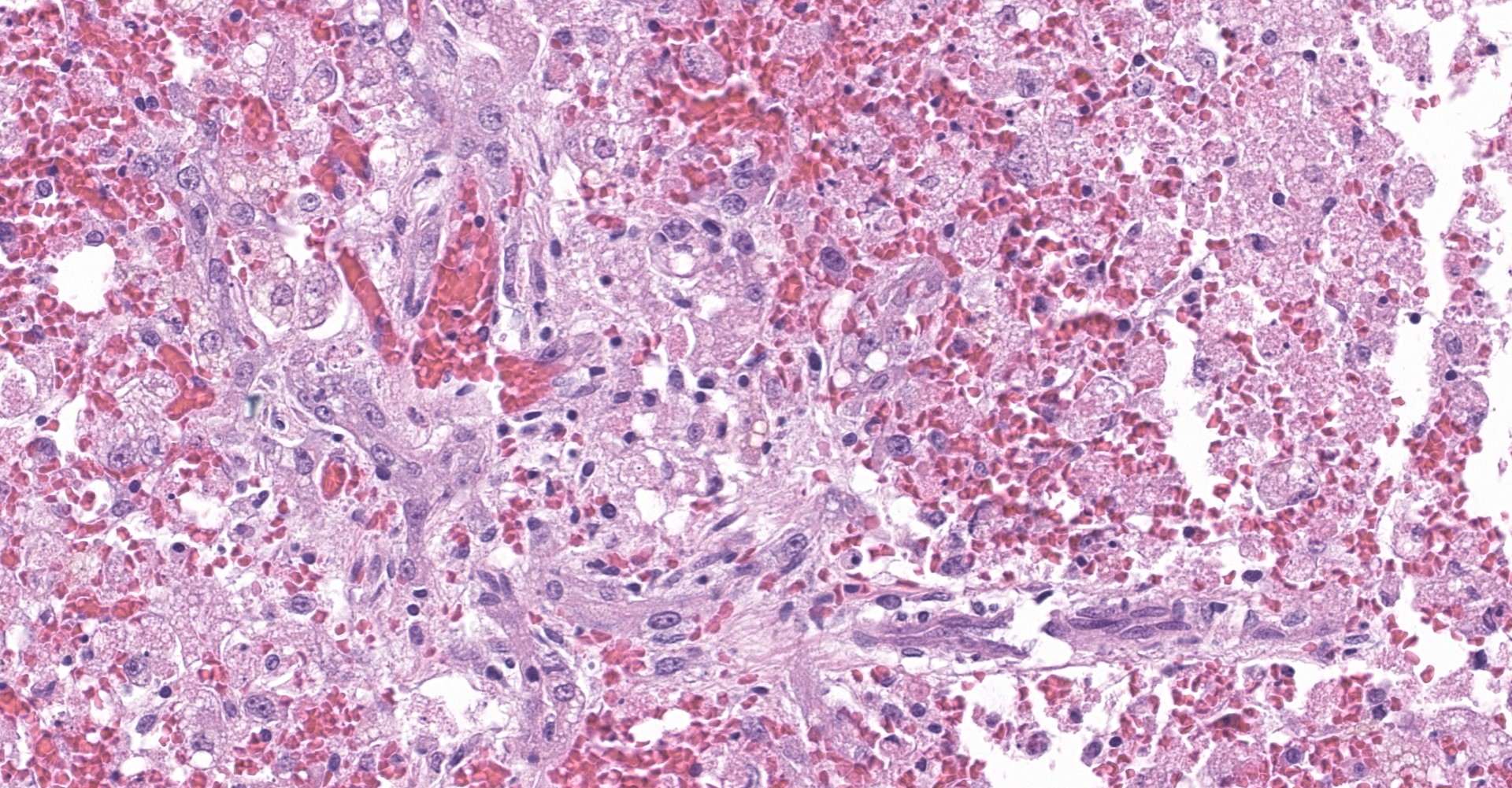
The slide contains one section of hepatic parenchyma in which massive necrosis is characterized by dissociation of hepatic cords to complete loss of hepatocytes across all zones of multiple lobules.
Lost hepatocytes are replaced by streams of extravasated red blood cells (hemorrhage), fibrin and karyorrhectic debris. Remaining individualized hepatocytes exhibit one or more of the following characteristics: cytoplasmic swelling, cytoplasmic vacuolation, cytoplasmic pigmentation, and nuclear pyknosis, karyorrhexis or karyolysis (degeneration and necrosis). Within the portal triads, portal collagen fibers are disrupted by increased clear space (edema), dissecting cords of hepatic progenitor cells (ductular reaction) and small numbers of lymphocytes and plasma cells. Scattered portal triads are additionally expanded by proliferating collagenous stroma (fibrosis) that breaches the limiting plate and spans portal regions (bridging portal fibrosis).
Contributor's morphologic diagnosis:
Liver: Massive hepatocellular necrosis with ductular reaction, hydropic degeneration, portal fibrosis and portal to portal bridging fibrosis
Contributor's comment:
The constellation of the patient's age, clinical history, bloodwork abnormalities and hepatic lesions were most concerning for a toxic etiology. Fresh samples of hepatic parenchyma were submitted for toxicological analysis. A trace of amanitin was detected, therefore confirming ingestion of Amanita phalloides, which is also known as the death cap or death angel mushroom.
Amanitin poisoning is classically divided into four consecutive clinical stages, although each stage may not be evident in each case. Following ingestion, the first stage is characterized by approximately 8 to 12 hours of no clinical abnormalities. The patient will then develop severe gastrointestinal signs including vomiting and bloody diarrhea. The third stage of toxicity is characterized by brief clinical improvement, referred to as a "false recovery", and quickly followed by the 4th stage, which is characterized by multi-organ failure. The liver and kidneys appear to be most severely affected within this fourth stage, as evidenced by striking derangements in bloodwork and urinalysis.
Patients typically die within 12 to 84 hours following ingestion of the lethal dose. The lethal dose (LD) for dogs is estimated to be around 0.5 mg/kg, which may be contained with 20 g of Amanita mushrooms.5 Therefore, one mushroom cap can provide a lethal dose to dogs. Evaluation of numerous treatment options has remained fruitless as the mortality rates in dogs remains high. Interestingly, studies have revealed remarkable variance in species sensitivity.4 For instance, the rate of gastrointestinal absorption is much greater in dogs than in mice and rabbits. Furthermore, rats appear to be relatively resistant to amanitin's toxic effects.
Amanitin's mechanism of action primarily stems from the toxin's ability to inhibit RNA polymerase II. Ceased transcription and decreased protein synthesis eventually leads to cell death. Cells with high metabolic rate, such as hepatocytes, crypt cells and proximal convoluted tubules, are the most severely affected, thus explaining the classic triad of hepatic failure, gastroenteritis and renal failure, respectively. In addition to inhibition of RNA polymerase II, amanitin may induce hepatocellular apoptosis, thus compounding hepatocellular death and hepatic failure.2
Given the patient's clinical history and the hepatic lesions, top differential diagnoses included blue-green algae poisoning (microcystins) and aflatoxin. However, no microcystins were detected within the patient's stomach content nor was any aflatoxin detected within the fresh samples of hepatic parenchyma.
Contributing Institution:
University of California
Davis Veterinary Medical Teaching Hospital
1 Garrod Drive
Attn: Anatomic Pathology Service
Davis, CA 95616
https://www.vetmed.ucdavis.edu/hospital
JPC diagnosis:
Liver, hepatocytes: Necrosis, massive, diffuse, acute, with hemorrhage and stromal collapse.
JPC comment:
The contributor provides an excellent overview of the pathogenesis and clinical progression of amanitin toxicosis following ingestion of Amanita phalloides.
A. phalloides is found worldwide due to the importation of trees from its native Europe. An ectomycorrhizal fungus, A. phalloides shares a symbiotic relationship with the roots of trees found in both deciduous and coniferous forests. In the United States the mushroom is most commonly found alongside oak trees on the west coast in contrast to the east coast where it is more commonly found alongside pine trees.1 The large fruiting bodies appear in the late summer and fall and have a smooth, yellowish-green to yellowish-brown cap, white gills, a white ring around the upper part of the stem (veil), and a white cup-like structure around the base of the stem (volva).4
Amatoxins are heat-stabile bicyclic octapeptides that include the amanitins (α-, β-, γ-, and ε-amanitins), amanin, amanullin, and proamanullin and have been isolated from several genera of mushrooms, including Amanita, Galerina and Lepiota.2,3 α-Amanitin is the most toxic of eight known amanitins and is found in 38 species of mushroom from the genera Amanita, including A. phalloides.1,2
Early diagnosis of amanitin toxicosis is frequently hindered as ingestion of the mushroom is often unwitnessed and veterinary care is not pursued until development of gastrointestinal signs or multi-organ failure.1 A recent study evaluating 59 cases of α-amanitin toxicosis in dogs found upon presentation, alanine transferase activity (ALT) was mildly to markedly increased in 97% of dogs, hypoglycemia in 78% and coagulation times were increased in 91%.1 Although the signs of liver failure are not specific to α-amanitin toxicosis, early onset hypoglycemia was found to be a distinguishing biochemical abnormality and its identification helped distinguish affected dogs from those with other causes of gastroenteritis; however, differential diagnoses should also include xylitol toxicosis, severe anaphylaxis, sepsis, and portosystemic shunt.1
During necropsy, the liver is often swollen, without any other significant abnormalities and is histopathologically characterized by massive hepatocellular necrosis with collapse of hepatic cords and acute tubular necrosis in dogs that develop renal failure. 4
Confirmatory diagnosis at select veterinary toxicology laboratories is made by detection of α-amanitin in submitted samples of serum, urine, gastric contents, suspected mushroom, liver or kidney. Serum and urine samples should be collected and frozen at various points beginning as early as possible following exposure.4
References:
1. Kaae JA, Poppenga RH, Hill AE. Physical examination, serum biochemical, and coagulation abnormalities, treatments, and outcomes for dogs with toxicosis from α-amanitin-containing mushrooms: 59 cases (2006-2019). J Am Vet Med Assoc. 2021;258(5):502-509.
2. Magdalan J, Ostrowska A, Piotrowska A, et al. Alpha-amanitin induced apoptosis in primary cultured dog hepatocytes. Folia Histochemica et Cytobiologica. 2010;48:58-62.
3. Puschner B, Rose HH, Filigenzi MS. Diagnosis of Amanita toxicosis in a dog with acute hepatic necrosis. J Vet Diagn Invest. 2007;19(3):312-317.
4. Puschner B, Wegenast C. Mushroom poisoning cases in dogs and cats: Diagnosis and treatment of hepatotoxic, neurotoxic, gastroenterotoxic, nephrotoxic and muscarinic mushrooms. Veterinary Clinics of North America: Small Animal Practice. 2012;42:375-387.
5. Spoerke David G., Rumack Barry H. Handbook of mushroom poisoning: diagnosis and treatment. Bosa Roca, United States: Taylor and Francis Inc.; 1994.
- Lewis HL, Kleiner DE. Hepatic injury due to drugs, herbal compounds, chemicals, and toxins. In: Burt AD, Ferrell LD, Portmann BC, eds. MacSween's Pathology of the Liver. 6th ed. Edinburgh: Churchhill Livingstone/Elsevier; 2012:690.