Signalment:
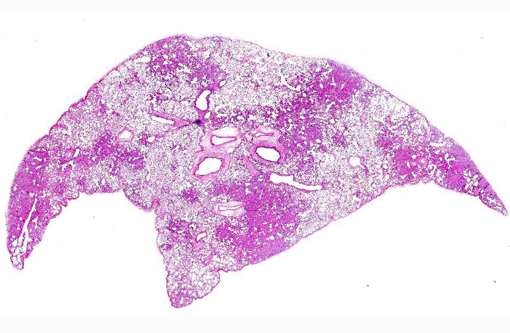
Gross Description:
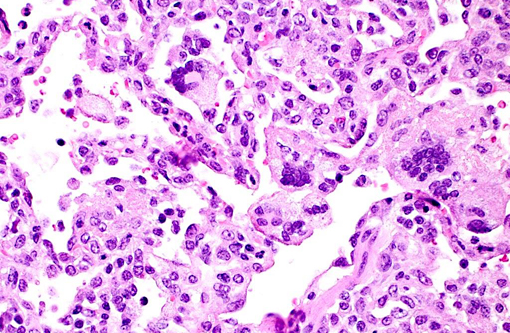
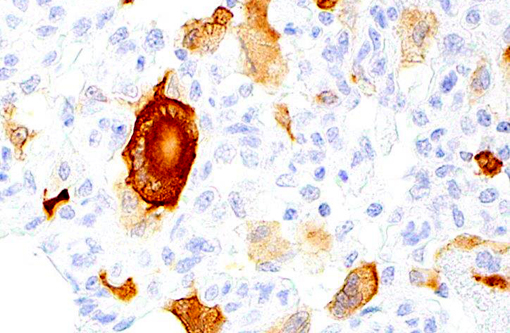
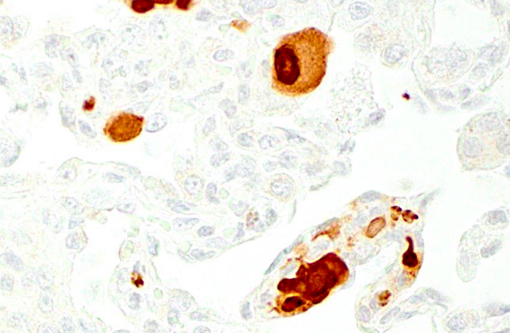
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
SIV belongs to genus lentivirus under the family Retroviridae. Retrovirus virions are enveloped, 80-100 nm in diameter, and have a unique three-layered structure. Innermost is the genome nucleoprotein complex, which includes about 30 molecules of reverse transcriptase, and has helical symmetry. This structure is enclosed within an icosahedral capsid, about 60 nm in diameter, which in turn is surrounded by a host cell membrane-derived envelope from which glycoprotein peplomers project. The genome is diploid, consisting of an inverted dimer of two molecules of linear positive-sense, single stranded RNA; each monomer is 7-11 kb in size and has a 3'-polyadenylated tail and a 5'-cap; the retrovirus genome is organized into 9 ORFs producing 15 proteins. SIV infection causes a spectrum of virally-induced lesions that include enteropathy, lymphocytic interstitial pneumonitis, giant cell pneumonia/lymphadenitis, and SIV encephalitis.Â
Giant cell pneumonia is characterized by multinucleated giant cells that are frequently found in terminal cases of SAIDS.(3) Formation of multinucleated giant cells is not completely defined but requires a myriad of factors including 1) infection with SIV, 2) cytokine elaboration from the multinucleated giant cells, and 3) macrophage infiltration.(2)
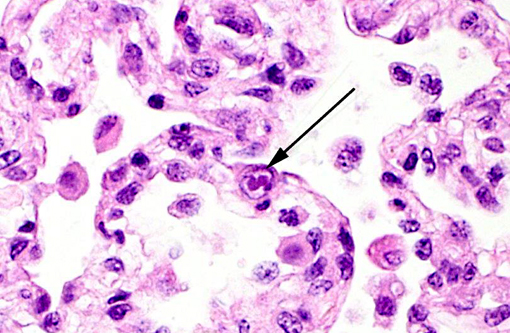
Cytomegalovirus (CMV) virions are enveloped, about 150 nm in diameter, and contain an icosahedral nucleocapsid about 100 nm in diameter. The genome consists of a single linear molecule of double-stranded DNA, 125-235 kbp in size. CMV belongs to the Betaherpesvirinae subfamily of Herpesviridae, under the order Herpesvirales. There are number of cytomegalovirus isolated from non-primates that include Cercopithecine herpesvirus 5 (CeHV-5) in African green monkeys, Cercopithecine herpesvirus 8 (CeHV-8) in rhesus monkeys, Human herpesvirus 5 (HHV-5) in humans, as well as Pongine herpesvirus in chimpanzees, Aotine herpesvirus 1 & Aotine herpesvirus 3 in owl monkeys. Betaherpesviruses replicate more slowly than alphaherpesviruses and often produce greatly enlarged cells, hence the designation cytomegalovirus.(16) Cytomegaloviruses infect the salivary glands, liver, spleen, lungs, eyes, and other organs, in which they produce characteristically enlarged cells with intranuclear inclusions and are typically accompanied by neutrophilic infiltrates.Â
Another common opportunistic lung infection in immunocompromised rhesus macaques is Pneumocystis. Pneumocystis pneumonia (PCP) is one of the most common opportunistic diseases in SAIDS.(1,2) Characteristic pathologic features of PCP include infiltration of inflammatory cells in the lung interstitium, thickened alveolar septa by hyperplastic type II pneumocytes, and foamy exudates in the alveoli. Some of these features were present in the submitted case; however, immunohistochemistry for Pneumocystis was negative in this case. Since Pneumocystis has a morphology similar to protozoa, it was initially considered as such; however, it is now classified as a fungus because the composition and structure of its cell wall (12,15) and nucleotide sequences are more similar to those of fungi than to those of protozoa.(6,13) Although Pneumocystis organisms are found in many different species of mammals, they are strictly species specific.(7) Some of the more common organisms include: Pneumocystis jirovecii (human), P. wakefieldii (rat), P. murina (mouse), and P. carinii (rhesus macaque).(5) In immunocompetent humans and animals, alveolar macrophages (AMs) protect the hosts against Pneumocystis infection by actively removing this extracellular organism from the alveoli. However, AMs from Pneumocystis infected animals are defective in phagocytosis,(4,9) and the number of AMs in humans and animals with PCP is reduced. These two defects impair innate immunity against Pneumocystis infection. The defect in phagocytosis is correlated to down regulation of mannose receptor on the macrophages.(8) The reduction in alveolar macrophage (AM) number is mainly due to increased rate of apoptosis(10) that is triggered by increased levels of intracellular polyamines(11) which could be due to either increased de novo synthesis and uptake of exogenous polyamines. Very little is known about the defect in phagocytosis during PCP.
JPC Diagnosis:
1. Lung: Pneumonia, interstitial, histiocytic, with numerous viral syncytial giant cells.Â
2. Lung, alveolar macrophages: Intranuclear viral inclusions, rare.
Conference Comment:
References:
1. Apetrei C, Robertson DL, Marx PA. The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front Biosci. 2004;9:225-254.
2. Baskar P, Narayan O, McClure HM, Hildreth JE. Simian immunodeficiency virus SIVsmmPBj 1.9 induces multinucleated giant cell formation in human peripheral blood monocytes. AIDS Res Hum Retroviruses. 1994;10:73-80.
3. Baskin GB, Murphey-Corb M, Martin LN, Soike KF, Hu FS, Kuebler D. Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1991;28:506-513.
4. Chen W, Mills JW, Harmsen AG. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int J Exp Pathol. 1992;73:709-720.
5. Durand-Joly I, Wakefield AE, Palmer RJ, Denis CM, Creusy C, Fleurisse L, et al. Ultrastructural and molecular characterization of Pneumocystis carinii isolated from a rhesus monkey (Macaca mulatta). Med Mycol. 2000;38:61-72.
6. Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HJ, Sogin ML. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988;334:519-522.
7. Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun. 1993;61:2886-2890.
8. Koziel H, Eichbaum Q, Kruskal BA, Pinkston P, Rogers RA, Armstrong MY, et al. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Invest. 1998;102:1332-1344.
9. Lanken PN, Minda M, Pietra GG, Fishman AP. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980;99:561-588.
10. Lasbury ME, Durant PJ, Ray CA, Tschang D, Schwendener R, Lee CH. Suppression of alveolar macrophage apoptosis prolongs survival of rats and mice with pneumocystis pneumonia. J Immunol. 2006;176:6443-6453.
11. Lasbury ME, Merali S, Durant PJ, Tschang D, Ray CA, Lee CH. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J Biol Chem. 2007;282:11009-11020.
12. Matsumoto Y, Matsuda S, Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989;36:21S-22S.
13. Pixley FJ, Wakefield AE, Banerji S, Hopkin JM. Mitochondrial gene sequences show fungal homology for Pneumocystis carinii. Mol Microbiol. 1991;5:1347-1351.
14. Silvestri G. AIDS pathogenesis: a tale of two monkeys. J Med Primatol. 2008;37 Suppl 2: 6-12.
15. Walker AN, Garner RE, Horst MN. Immunocytochemical detection of chitin in Pneumocystis carinii. Infect Immun. 1990;58:412-415.
16. Yue Y, Barry PA. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res. 2008;72:207-226.