Wednesday Slide Conference, Conference 17, Case 3
Signalment:
Juvenile, female Angus cow, bovine (Bos taurus)
History:
This cow was one of 45 presenting with cutaneous lumps when mustered from a herd of 95 animals. Rectal temperatures ranged from 40-41ºC and some of the affected cows appeared sick. Some cows were panting with increased lung sounds. The mucous membranes were pink. There was no evidence of typical photosensitization lesions in predisposed locations.
Gross Pathology:
Nodules were distributed over the neck, brisket, thorax and to a lesser extent abdomen and forelimbs. Nodules ranged from 10-30mm diameter and were not ulcerated, although the surface of some could be scratched off.
Laboratory Results:
A Pan-Herpesvirus nested PCR was performed, and the product was sent for Sanger sequencing. A BLAST query of the product sequence found it was 100% identical to Bovine alphaherpesvirus 2 [MT862164] 181 nt partial sequence. A ruminant biochemistry panel found elevation in GLDH 96 U/L (0-30 U/L), mild hyperglobulinaemia 48.4 g/L (30.0-45.0 g/L) and mild elevation in serum hemoglobin 0.25 g/dL (0.00-0.20 g/dL). A Capripoxvirus TaqMan assay on the EDTA blood and a skin scab was negative, and a Capripox double antigen multispecies ELISA performed on the serum sample was negative.
Microscopic Description:
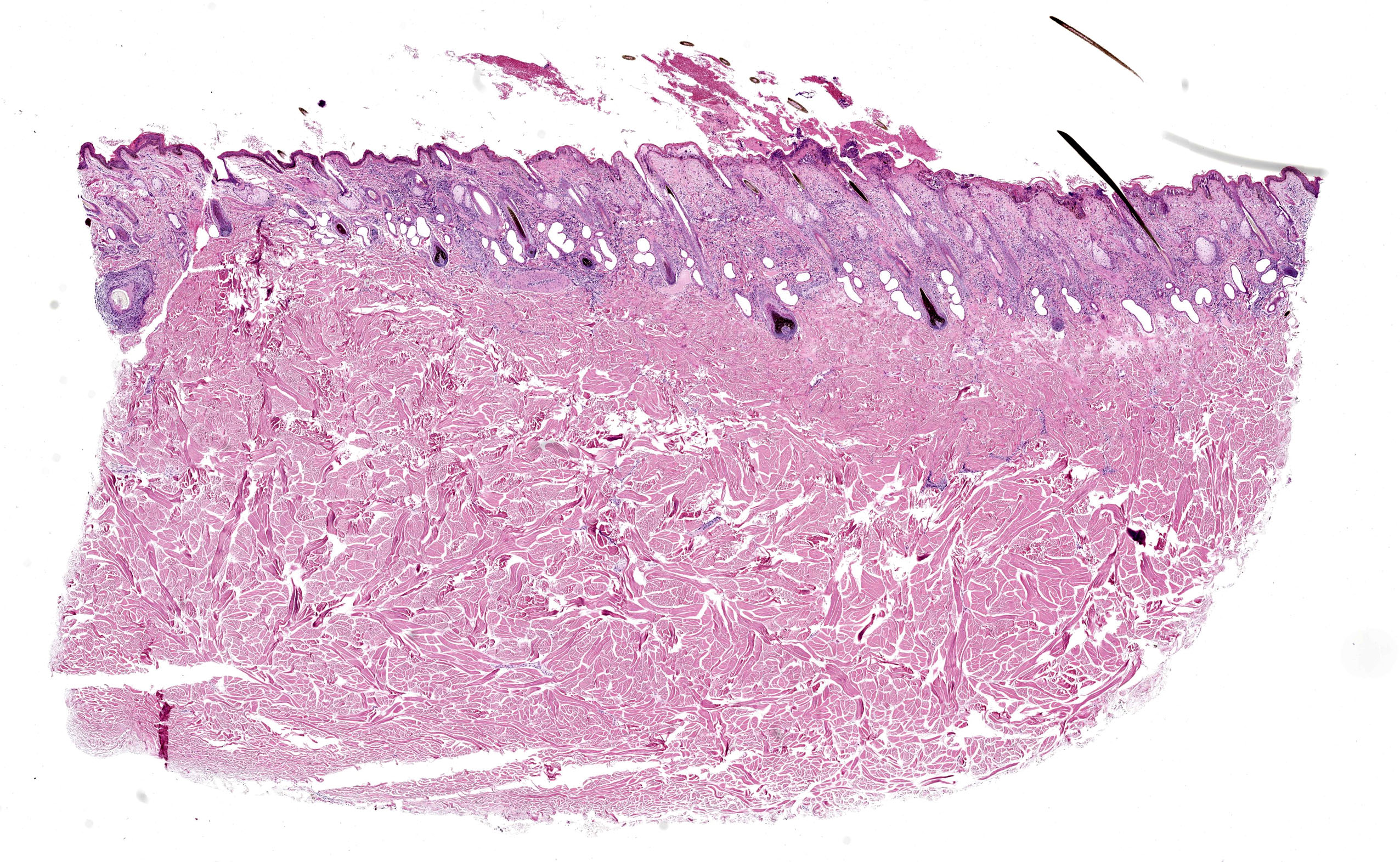
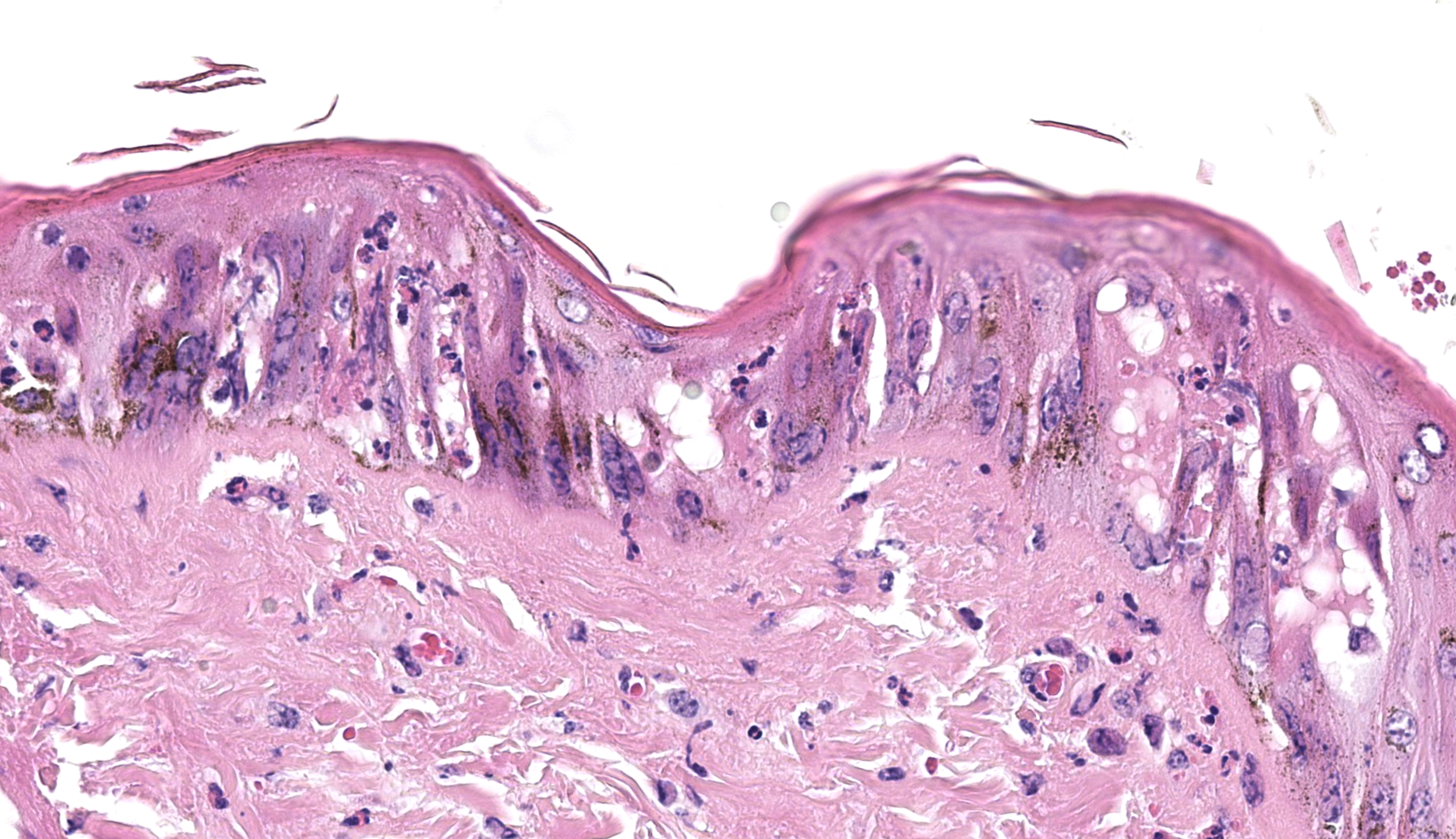
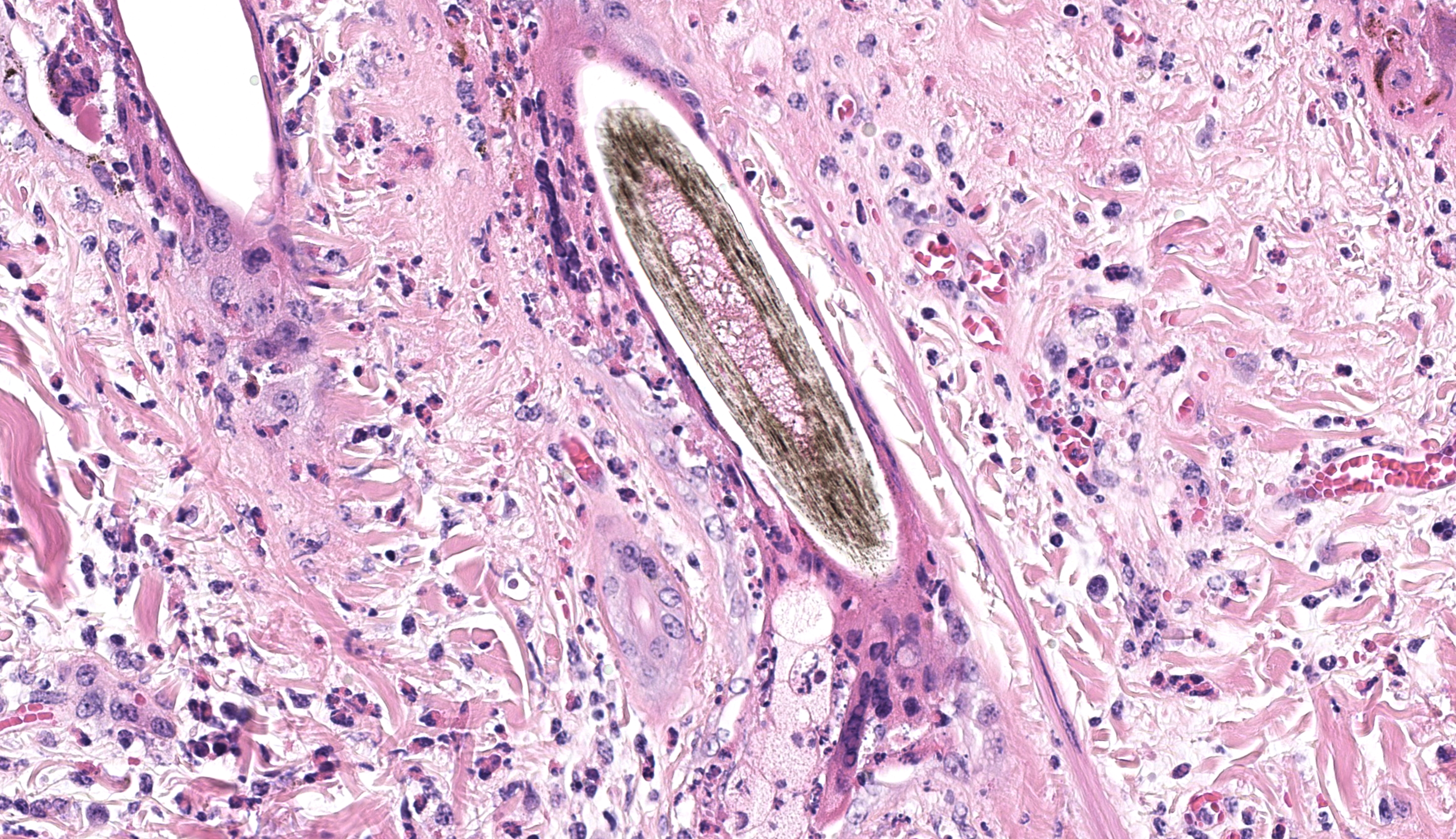
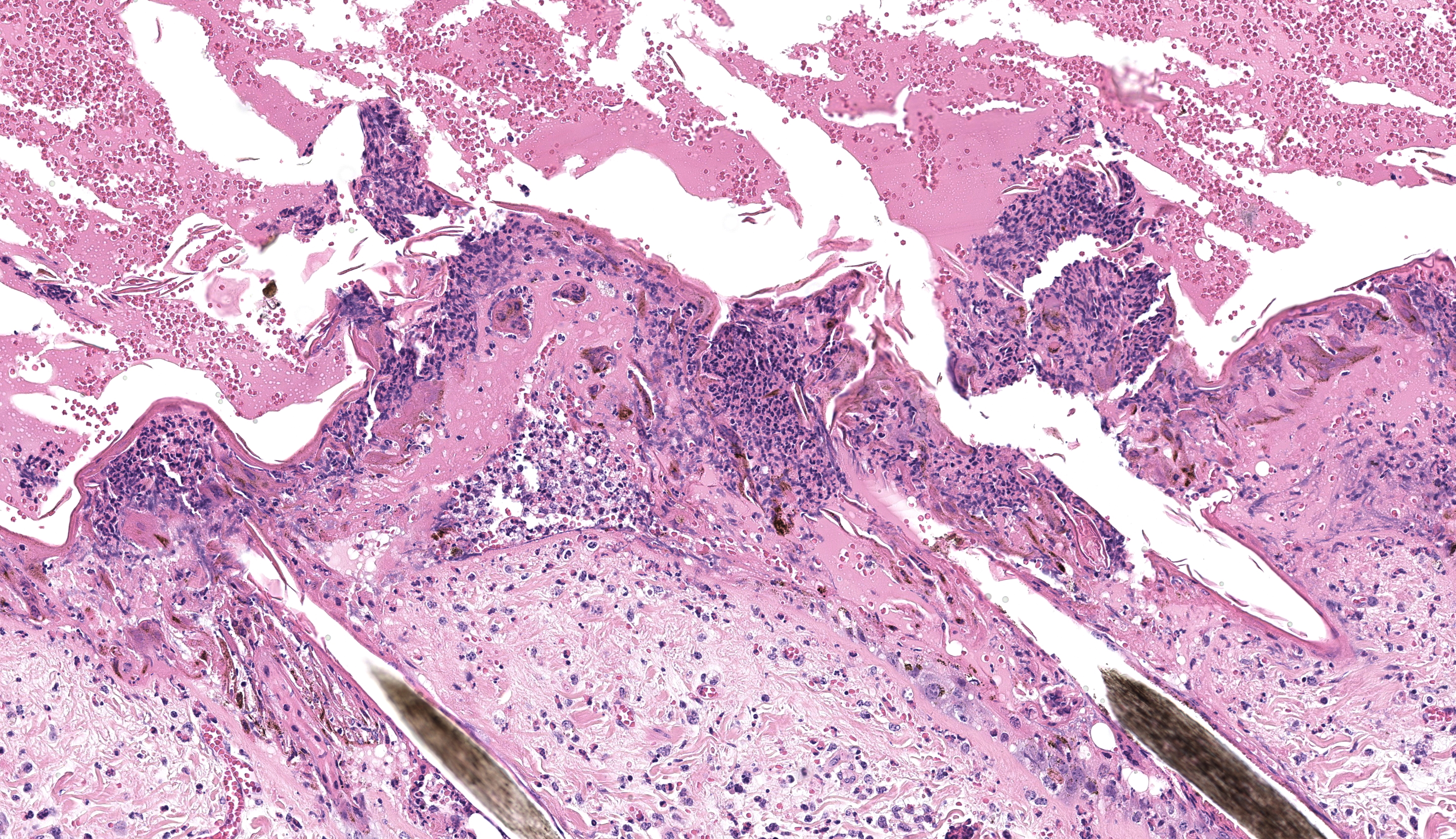
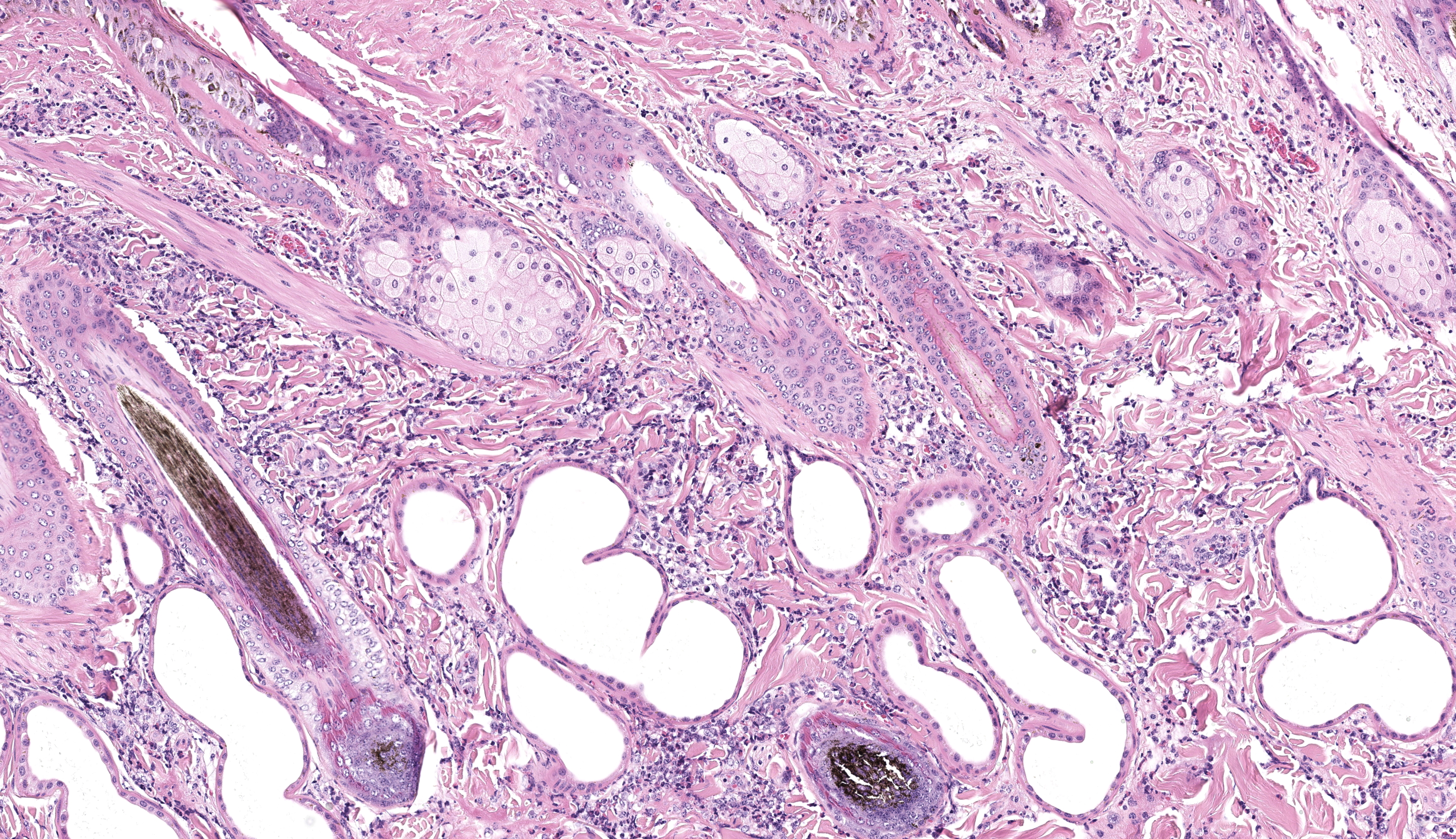
Haired skin (x3): Within the sections of haired skin, multifocally within the epidermis, there are hypereosinophilic necrotic keratinocytes, numerous small expansions containing eosinophilic fluid (vesicles) and necrotic cell debris and degenerate neutrophils, sometimes forming small aggregates (micropustules). Multifocally, epithelial cells within the stratum basale, stratum spinosum and stratum granulosum are sloughed, replaced with clumps of multinucleated epithelial cells with hypereosinophilic cytoplasm (syncytia). Moderate numbers of epithelial cells and syncytia within the surface epithelium, and occasionally within epithelium lining hair follicles, contain 1-6µm diameter intranuclear eosinophilic inclusion bodies with margination of chromatin. Multifocally, keratinocytes are swollen with clearing of cytoplasm (ballooning degeneration). There is mild, multifocal orthokeratotic hyperkeratosis, with mild to moderate extravasated erythrocytes adjacent to the epidermal surface (hemorrhage). Throughout the superficial and deep dermis, there are multifocal, moderate infiltrates of neutrophils, lymphocytes, plasma cells, eosinophils and macrophages associated with cell debris, and multifocal mild to moderate separation of dermal collagen fibres with clear space and/or eosinophilic fluid (edema). Occasionally sebaceous glands are surrounded and infiltrated by neutrophils and apocrine glands are dilated. Few lymphatic vessels in the deep dermis are dilated. Within the dermis of one section, adjacent to the cut margin, there is a focal aggregate of refractile material, surrounded by eosinophilic necrotic material, and subsequently surrounded by a moderate margin of leukocytes with squash artefact.
Contributor’s Morphologic Diagnosis:
Haired skin, dermatitis, necrosuppurative and, lymphoplasmacytic multifocal to widespread, chronic-active, with edema, syncytial cells, pustules, vesicles and intranuclear epithelial eosinophilic viral inclusion bodies, Bos taurus.
Contributor’s Comment:
Infection with Bovine Herpesvirus 2 (BoHV-2) in cattle can cause two distinct clinical conditions: one is an acute onset, mild generalized skin infection called Pseudo Lumpy Skin Disease (PLSD) which manifests as cutaneous nodules which develop a central depression; the second is Bovine Herpesvirus Mammillitis (BHM), a localized ulcerative condition affecting the teats and udder of cattle. The disease is found in many parts of the world and is likely transmitted by biting insects or mechanically by equipment used on farms. The gross appearance of the generalized skin lesions can closely resemble Lumpy Skin Disease, which has trade implications in many countries.21
Bovine herpesvirus 2 (BoHV-2) is an enveloped double stranded DNA virus approximately 150-200nm in size, belonging to the family Orthoherpesviridae, subfamily Alphaherpesvirinae, genus simplexvirus.
Bovine herpesvirus 2 infection was first identified in Southern and Eastern Africa. Since its discovery in the 1950’s, it has been reported almost globally with case reports from Europe, Japan, Australia, Brazil, the United States and Israel.2,3,8–10,13,16
In the wild, BoHV-2 infects cattle, wild ruminants and pseudo-ruminants (hippopotamus).13 Sheep have been experimentally infected with BoHV-2 producing clinical disease similar to Bovine Herpesvirus Mammillitis.17 Rabbits, guinea pigs and mice have also been experimentally infected with BoHV-2.8
Transmission of BoHV-2 most likely occurs via intradermal injection of the virus by biting insects, or via contaminated equipment coming into contact with wounds and cuts. Biting flies have been demonstrated to be able to transmit the infection between cell cultures in the lab, and the distribution and seasonality of outbreaks of Pseudo Lumpy Skin Disease are thought to be related to the distribution and peaks in biting insect activity.6,9,12 Milking equipment is suspected of being able to transmit the virus within milking herds if clinically affected cattle are present.5,10
Serological surveys indicate that circulation of BoHV-2 in cattle can occur without any episodes of clinical disease suggesting most infections may be subclinical.8,9,15
In experiments where BoHV-2 was intradermally inoculated into the flank of cattle, a discrete raised swelling was observed at the injection site by day 3 post inoculation. Generalized discrete swellings appeared over the head, neck and dorsal body by day 6 which developed crusts which gradually lifted over the course of 3 weeks to reveal greyish-white areas of skin with reduced numbers of hairs (alopecia). Lymph node enlargement was observed by day 5 and persisted to day 10 post inoculation.16
In the field, the incubation period of BoHV-2 is considered to be around 1-2 weeks, with skin lesions resolving 2-4 weeks after first appearing. Neutralizing antibodies appear 7-14 days after initial exposure.16,18 Outbreaks can occur seasonally or sporadically depending on the activity of biting insects and the presence of naive hosts.11,19
Post infection, like other herpesviruses, BoHV-2 becomes latent with the virus likely retreating to the neuronal tissue such as the trigeminal ganglion and possibly lymphoid tissue where it may lay dormant until infection is reactivated during periods of stress.3,17
Generalized infection with BoHV-2 induces Pseudo Lumpy Skin Disease, which is characterized by:1,4,16
- Circumscribed cutaneous nodules over the body, frequently on the head, neck, shoulders, back and perineum.
- Around 4-6 days after the appearance of gross lesions, the cutaneous lesions begin to crust over. The crusts eventually fall off leaving areas of alopecia.
- Lesions disappear when the hair regrows.
- Lymphadenitis may be observed during early stages of infection.
- Animals make a full recovery without treatment.
Localized infection with BoHV-2 of the udder and teats is called Bovine Herpesvirus Mammillitis, and is characterized by:11,20
- Variable sized tender edematous swellings on the udder or teats.
- Vesicles may appear on affected areas
- The overlying skin then ulcerates and becomes painful, taking 3-10 weeks to heal
Histopathological changes of BoHV-2 infection are characterized by the formation of epithelial syncytia initially within the stratum basale and inner stratum spinosum with prominent intranuclear eosinophilic inclusion bodies.11 Intranuclear inclusions are classified as Cowdry type A inclusions and have central eosinophilic or amphophilic deposits surrounded by a clear halo and the nuclear chromatin is pushed to the margin of the nucleus.19 Intranuclear inclusions are most numerous after the appearance of the gross nodules but before the appearance of crusts (around 4-6 days later),1,11 which coincides approximately with the time when virus can be isolated or detected by PCR (see below). When the lesions crust over, the epithelium becomes necrotic and infiltrated by large numbers of neutrophils. Loss of the necrotic epithelium results in an ulcer which becomes covered with haemorrhage, fibrinous exudate and numerous degenerate neutrophils. Deeper in the dermis, there is edema and a mononuclear infiltrate. As the lesion heals, there is fibrosis in the superficial dermis with perivascular infiltrates of mononuclear cells.11
The following assays have been reported in the literature to support the diagnosis of BoHV-2 infection.
|
Test |
Required Samples |
Notes |
|
PCR1,12,19 |
Fresh skin lesions |
Most likely to yield detectable virus DNA up to 8 days post infection or before cutaneous lesions crust over or ulcerate |
|
Cytology11 |
Early skin lesions (<5 days after initial appearance) |
Presence of syncytia with intranuclear inclusions |
|
Serology - ELISA15 |
Serum |
|
|
Serology – Serum neutralization9,18 |
Serum |
Antibodies detected as early as 10 days post inoculation or about 5-7 days after appearance of cutaneous lesions |
|
Serology – Immunofluorescent antibody test (IFAT)10 |
Serum |
|
|
Negative staining electron microscopy19 |
Fresh skin lesions |
Herpesvirus particles may be seen |
|
Virus culture6,9,10,16,18 |
Blood, Fresh skin lesions up to around day 6-7 post infection |
Vero cells Calf kidney cells Fetal bovine muscle cells Madin Darby Bovine Kidney (MDBK) |
The following conditions should be considered as potential differentials for Bovine Herpesvirus-2 infection based on their clinical appearance.4,11,20
|
Disease |
Clinical Presentation |
Tests to diagnose/ differentiate |
Key differentiating histological features |
|
Viral |
|
|
|
|
Early-stage Lumpy Skin Disease (Capripoxvirus) |
Firm circumscribed cutaneous nodules which develop central areas of necrosis and scarring |
Capripox PCR, biopsy + histopathology, Electron microscopy demonstrating pox virus particles |
Epidermal vacuolar swelling with intracytoplasmic inclusions. Vasculitis |
|
Bovine Papillomavirus |
Papillomas or warts of varying size on body. Mostly frequently seen in younger cattle |
Biopsy + histopathology, PCR of skin lesions |
Unencapsulated, proliferative cutaneous neoplasm accompanied by basal cell hyperplasia, acanthosis and hyperkeratosis |
|
Bovine papular stomatitis (Parapoxvirus) |
Raised red papules, erosions or ulcers around muzzle and nose, occasionally elsewhere on the body |
Electron microscopy of tissue, virus culture, biopsy + histopathology, ELISA/Agar gel precipitation tests to look for antibodies |
Epidermal vacuolar swelling with intracytoplasmic inclusions, epidermal proliferation |
|
Pseudocowpox (Parapoxvirus) |
Raised red sores, scabs or vesicles on teats, thighs or perineum |
PCR on skin lesions, scabs or blood, biopsy + histopathology. |
Epidermal vacuolar swelling with intracytoplasmic inclusions, epidermal proliferation |
|
Cowpox (Orthopoxvirus) |
Papules or ulcers on the teat or udder |
PCR of skin lesions, electron microscopy of scabs demonstrating pox virus particles14, biopsy + histopathology |
Epidermal vacuolar swelling with intracytoplasmic inclusions, focal epidermal hyperplasia. |
|
BVDV dermatitis |
Patchy hyperkeratosis around neck, shoulder and perineum |
Bovine Viral Diarrhea Virus Antigen ELISA or PCR on ear notch, blood, hair or visceral organs |
|
|
Bovine Herpesvirus 4 |
Vesicles, pustules and ulcers on udder. Teats are usually not involved |
PCR on skin lesions, serological testing, biopsy + histopathology |
Intraepithelial pustular dermatitis |
|
Bacterial |
|
|
|
|
Dermatophilosis (Dermatophilus congolensis) |
Papules with crusts entrapped in hair and areas of alopecia often over the dorsal aspect of head, neck and body |
Bacterial cultures, cytology of crusts, biopsy + histopathology |
Multiple parallel rows of cocci, trapped within alternating layers of hyperkeratosis and serous fluid and degenerate neutrophils. Purulent folliculitis |
|
Cutaneous tuberculosis (Mycobacterium bovis) |
Single or multiple nodules, abscesses or ulcers |
PCR, mycobacterial culture, biopsy + histopathology |
Pyogranulomatous dermatitis with central caseous necrosis with acid fast bacilli present. |
|
Fungal |
|
|
|
|
Dermatophytosis (Ringworm, Trichophyton verrucosum) |
Expanding circular hairless lesions mostly on head and neck. May appear red and moist to scaly and grey. |
Dermatophyte cultures, biopsy + histopathology, PCR |
Fungal hyphae clustering within hair shafts and arthrospores around the outside of hairs. |
|
Parasites |
|
|
|
|
Mites (multiple species) |
Nodules, alopecia and crusts which may be itchy |
Superficial or deep skin scrapings |
|
|
Onchocercosis |
Cutaneous nodules over brisket, abdomen and udder |
Biopsy + histopathology |
Presence of microfilaria, surrounded by eosinophilic inflammation |
|
Besnoitiosis (Besnoitia sp.) |
Alopecia, scaling and thickening of skin over neck ,shoulders and rump |
biopsy + histopathology, PCR, serology |
Presence of protozoal tissue cysts |
|
Hypoderma sp. (warbles) |
Firm raised nodules in the skin frequently along the back and shoulders |
Clinical examination and presence of breathing holes Serological tests |
|
|
Neoplasia |
|
|
|
|
Cutaneous Lymphoma |
Multifocal subcutaneous/intracutaneous nodules, often accompanied by alopecia, lymphadenopathy |
biopsy + histopathology, haematology may reveal lymphocytosis. May arise sporadically and not necessarily be associated with bovine leukosis virus infection |
Sheets of neoplastic lymphocytes. |
|
Mast cell tumor |
Mulltifocal cutaneous or subcutaneous nodules over body +/- alopecia +/- pigmented +/- ulceration |
biopsy + histopathology |
Sheets of neoplastic mast cells often accompanied by numerous eosinophils |
|
Other |
|
|
|
|
Photosensitization |
Alopecia, erythema and blistering of non-pigmented skin |
History – exposure to toxic plants, drug administration, biochemistry – liver function, biopsy + histopathology |
Coagulative necrosis of epidermis, dermal edema, vascular necrosis |
|
Skin allergies/urticaria |
Haired wheals +/- crusts anywhere on the body, angioedema |
History of recent drug administration, insect or plant exposure, response to glucocorticoid administration, biopsy + histopathology |
Dermal edema, perivascular infiltrates of granulocytes, mast cells and lymphocytes inconsistently present in upper to mid dermis. |
Contributing Institution:
Elizabeth Macarthur Agricultural Institute, Department of Primary Industries and Regional Development
JPC Diagnosis:
Haired skin: Dermatitis, necrotizing, subacute, diffuse, severe, with epithelial viral syncytia, intranuclear viral inclusions, corneal pustules, mixed dermal infiltrates, and edema.
JPC Comment:
The contributor provides an excellent section to accompany an thorough writeup on the topic. The slide has generous viral inclusions and syncytia along with raised vesicles to establish an etiological diagnosis. Conference participants did touch on the nature of intracellular and intercellular edema in this case the lack of overt cellular swelling and intracytoplasmic inclusions lowered suspicion for a poxviral etiology.
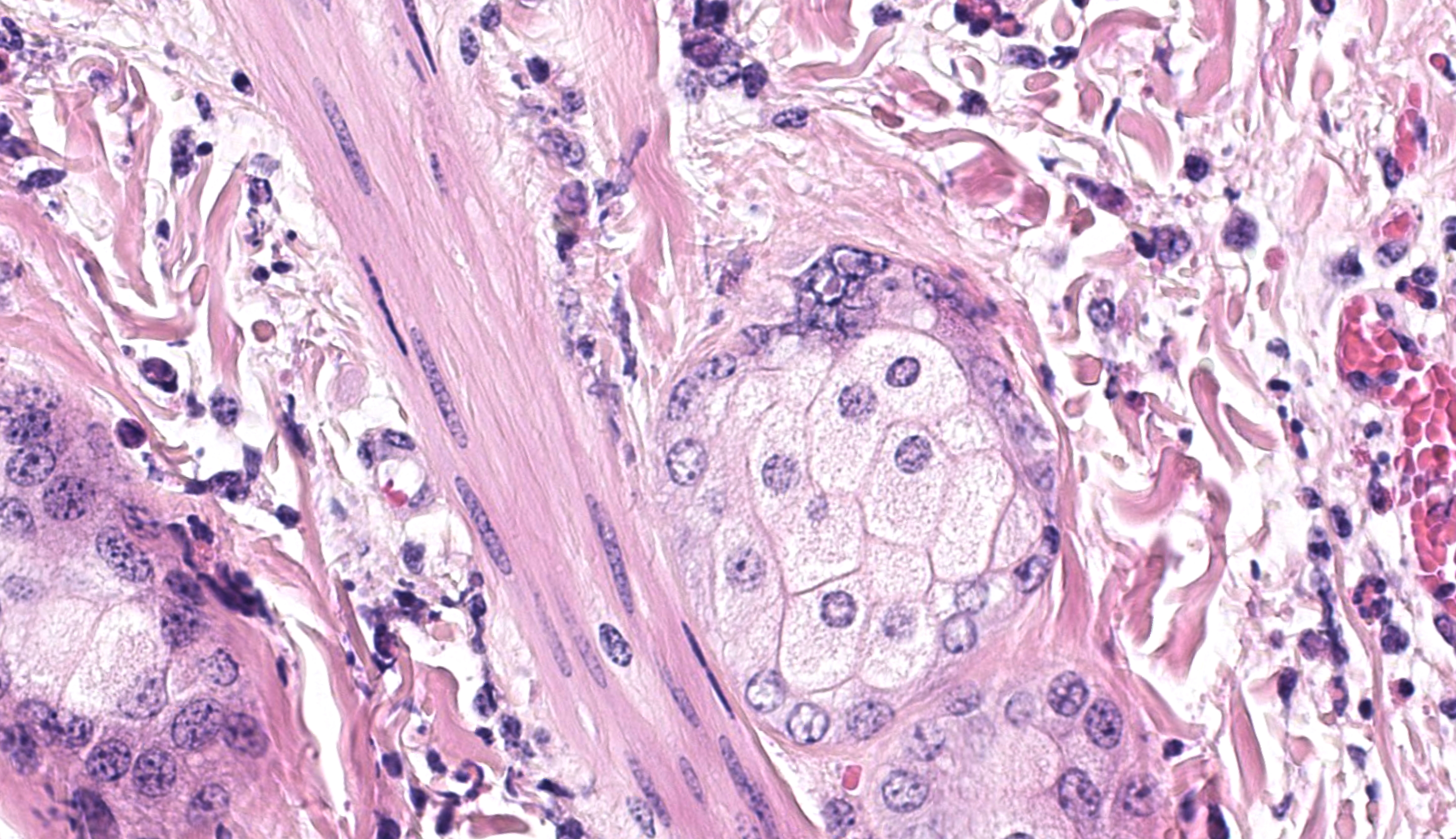
One ancillary finding in section was a focal granuloma centered on foreign material (possibly plant material). We speculated that this might be secondary to necrosis of the overlying epithelium and entry into the dermis, though the adjacent portions of skin lack significant viral changes. This lesion is at the edge of the section and is only on one of the three sections of skin provided which limits further interpretation of this incidental observation.
References:
- Amaral B, Dos Santos Jardim J, Cargnelutti J, Martins M, Weiblen R, Flores E. Pathogenesis of Bovine alphaherpesvirus 2 in calves following different routes of inoculation. Pesqui Veterinária Bras. 2020;40:360–367.
- Brenner J, Sharir B, Yadin H, Perl S, Stram Y. Herpesvirus type 2 in biopsy of a cow with possible pseudo-lumpy-skin disease. Vet Rec. 2009;165(18):539–540.
- Campos FS, Franco AC, Oliveira MT, et al. Detection of bovine herpesvirus 2 and bovine herpesvirus 4 DNA in trigeminal ganglia of naturally infected cattle by polymerase chain reaction. Vet Microbiol. 2014;171(1):182–188.
- Department of Agriculture, Fisheries and Forestry. Differential diagnoses for lumpy skin disease. Australian Government 2023:
- Gibbs EPJ, Johnson RH, Osborne AD. Experimental Studies of the Epidemiology of Bovine Herpes Mammillitis. Res Vet Sci. 1973;14(2):139–144.
- Gibbs EPJ, Jonnson RH, Gatehouse AG. A Laboratory Technique for Studying the Mechanical Transmission of Bovine Herpes Mammillitis Virus by the Stable Fly (Stomoxys calcitrans L.). Res Vet Sci. 1973;14(2):145–149.
- Hulo C, de Castro E, Masson P, et al. ViralZone: a knowledge resource to understand virus diversity. Nucleic Acids Res. 2011;39(Database issue):D576-582.
- Huygelen C, Thienpont D, Dekeyser PJ, Vandervelden M. Allerton Virus, a Cytopathogenic Agent Associated with Lumpy Skin Disease. Zentralblatt Für Veterinärmedizin. 1960;7(8):754–760.
- Imai K, Ishihara R, Nishimori T. First demonstration of bovine herpesvirus 2 infection among cattle by neutralization test in Japan. J Vet Med Sci. 2005;67(3):317–320.
- Lanave G, Larocca V, Losurdo M, et al. Isolation and characterization of bovine alphaherpesvirus 2 strain from an outbreak of bovine herpetic mammillitis in a dairy farm. BMC Vet Res. 2020;16(1):103.
- Mauldin EA, Peters-Kennedy J. Chapter 6 - Integumentary System. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 1 (Sixth Edition). W.B. Saunders 2016:509-736.e1.
- d’Offay JM, Floyd JG, Eberle R, et al. Use of a polymerase chain reaction assay to detect bovine herpesvirus type 2 DNA in skin lesions from cattle suspected to have pseudo-lumpy skin disease. J Am Vet Med Assoc. 2003;222(10):1404–1407, 1366–1367.
- Plowright W, Jessett DM. Investigations of Allerton-type herpes virus infection in East African game animals and cattle. J Hyg (Lond). 2009/05/15 ed. 1971;69(2):209–222.
- Shivanna V, Cino-Ozuna AG, Heskett C, Marthaler DG, Ganta C. Pseudocowpox virus infection in an American bison (Bison bison). BMC Vet Res. 2020;16(1):241.
- Singer S, Hoffmann B, Hafner-Marx A, et al. Bovine Alphaherpesvirus 2 infections in Bavaria: an analysis of the current situation - several years after eradicating Bovine Alphaherpesvirus 1. BMC Vet Res. 2020;16(1):149.
- St George TD, Uren MF, Melville LF. A generalised infection of cattle with bovine herpesvirus 2. Aust Vet J. 1980;56(1):47–48.
- Torres FD, Almeida SR, Silva MS, Weiblen R, Flores EF. Distribution of latent bovine herpesvirus 2 DNA in tissues of experimentally infected sheep. Res Vet Sci. 2009;87(1):161–166.
- Turner AJ, Kovesdy L, Morgan IR. Isolation and characterisation of bovine herpesvirus mammillitis virus and its pathogenicity for cattle. Aust Vet J. 1976;52(4):166–169.
- Watanabe TTN, Moeller RB, Crossley BM, Blanchard PC. Outbreaks of bovine herpesvirus 2 infections in calves causing ear and facial skin lesions. J Vet Diagn Invest. 2017;29(5):686–690.
- White SD, Théon AP, Angelos JA, Makhdoomi MM. Chapter 40 - Diseases of the Skin. In: Smith BP, Van Metre DC, Pusterla N, eds. Large Animal Internal Medicine (Sixth Edition). Mosby 2020:1316-1351.e11.
- World Organisation for Animal Health. Infection with lumpy skin disease virus. In: Terrestrial Animal Health Code. World Organisation for Animal Health (OIE) 2024: