Signalment:
Six-month-old, male, Tonkinese cat, (
Felis catus).The cat
presented for further investigation of a vestibular ataxia, head tremors, inappetence,
and lethargy. The cat was in poor body condition compared to its littermate and
had previously been found to be pyrexic. Neurological examination findings
were consistent with a central vestibular syndrome. MRI of the brain revealed
marked dilatation of the third and fourth ventricles and mild dilatation of the
lateral ventricles. There was marked contrast enhancement of the ependymal
lining and meninges, consistent with feline infectious peritonitis and
obstructive hydrocephalus. Cerebellar herniation was present with caudal
displacement of the cerebellar vermis through the foramen magnum, consistent
with elevated intracranial pressure. A provisional diagnosis of feline
infectious peritonitis (FIP) was made. Due to a grave prognosis, the cat was
euthanized and submitted for necropsy examination.
Gross Description:
An increased
volume of clear fluid drained from the cranium as the brain was removed and
there appeared to be diffuse flattening of the cortical gyri. The brain was
dissected following fixation and there was moderate dilation of the third,
fourth and lateral ventricles.
Histopathologic Description:
Brain
: Sections of
forebrain, midbrain, cerebellum and brainstem are examined. Variably
within the examined sections the meninges, choroid plexus and ependyma are
multifocally and extensively expanded by dense infiltrates of lymphocytes,
plasma cells, macrophages and rare viable and degenerate neutrophils. There is
prominent perivascular cuffing, predominantly targeting veins, of
periventricular and meningeal blood vessels by lymphocytes, macrophages, plasma
cells and occasional viable and degenerate neutrophils. The inflammatory
infiltrate extends into both vascular walls and beyond the Virchow-Robin spaces
into the adjacent neuropil. There is a mild to
moderate gliosis within the adjacent neuropil and periventricular white
matter contains variably-sized extracellular clear spaces (edema). Ependymal cells lining the ventricles appear
mildly elongate with disruption and increased spacing between cells.
Multifocally, there is necrosis of the ventricular lining with deposition of
fibrin.
Immunohistochemistry
Immunohistochemical
labeling for feline coronavirus antigen identified scattered, large, foamy
round cells (macrophages) which labeled positively for Coronavirus antigen
within the lesions in the brain tissue.
Morphologic Diagnosis:
1. Brain: multifocal, marked, pyo-granulomatous
and lymphoplasmacytic meningoencephalitis with vasculitis and perivasculitis,
ventriculitis, choroiditis and ependymitis
2. Brain:
moderate acquired hydrocephalus
Lab Results:
Feline
coronavirus antibody titer was extremely high; CSF (post mortem) showed a
marked, mixed pleocytosis with neutrophilic predominance and markedly increased
protein con-centration.
Condition:
Pneumonia/Acanthamoeba spp
Contributor Comment:
Feline
corona-viruses (FCoVs) are enveloped,
single-stranded, positive-sense RNA viruses that belong to the Coronaviridae
family, Alphacoronavirus genus and exist as two pathotypes, feline
infectious peritonitis virus (FIPV) and feline enteric coronavirus (FECV).1,3
Despite FCoVs being ubiquitous in the environment, with a prevalence of more
than 90% of cats in multicat households, feline infectious peritonitis (FIP) is
sporadic, with young, entire male, purebreed cats most commonly affected1.
Feline enteric coronavirus is generally considered avirulent, however, can be
associated with hemorrhagic enteritis and diarrhoea and affected cats may become persistently infected and continue to shed virus.
In contrast, FIPV results in severe, systemic disease and is a common cause of
neurologic disorders in young cats. Feline
coronavirus is generally transmitted via the fecal-oral route following which
it infects enterocytes, eventually being restricted to the caecum and colon.1
Analysis of
FECVs and FIPVs suggests a complex scenario involving several gene mutations to
result in increased virulence1,3. Feline
infectious peritonitis viruses appear to have an increased ability to replicate
in macrophages and monocytes and result in systemic disease.1
Virus-infected
macro-phages localize to small and medium sized veins within the serosa and
damage endothelial cells with the subsequent immune response resulting in a
vasculitis. A strong cell-mediated response is protective against FIP, whereas
a weak cell-mediated response results in the dry form of the disease. The
wet form of disease results from a lack of cell-mediated immune response to
the virus. Both type III and type IV hypersensitivity responses have been
implicated in the pathogenesis of FIP.5
Feline
infectious peritonitis is often distinguished by a wet or effusive form and a
dry non-effusive form with a proportion of cases showing a combination of the
two. Typical gross findings associated with the effusive form of FIP include a
fibrinous and granulomatous peritonitis, protein rich effusions and visceral
granulomatous inflammatory foci. In 60% of non-effusive cases of FIP there is
involvement of the eyes and/or CNS with or without granulomatous lesions in the
thoracic and abdominal viscera and frequently in the absence of a grossly
apparent peritonitis2. Gross lesions
in the brain are often unapparent, however may include thickening and opacity
of the meninges, and an obstructive hydrocephalus, as seen in this case.
Proteinaceous material within the ventricular system may be visible as
grey-blue gelatinous material.4
Clinical signs
associated with FIP are often vague and include pyrexia, lethargy and
inappetence with additional changes dependent on the distribution of tissues
affected.2
The
characteristic microscopic findings associated with FIP include a vasculitis
and perivasculitis, predominantly affecting small to medium sized venules. A high
proportion of macrophages alongside variable numbers of neutrophils,
lymphocytes and plasma cells infiltrate and surround vessels. Vascular necrosis
with thrombosis and infarction may also occur.2
Feline
infectious peritonitis may be suspected based on the signalment, compatible
clinical signs, and identification of pathognomonic gross and histologic
lesions. Identification of viral antigen in lesions using immuno-histochemistry
or real time RT-PCR is confirmatory for a diagnosis of FIP.3
JPC Diagnosis:
Cerebrum,
brainstem: Meningoencephalitis, lymphoplasmacytic and histiocytic,
perivascular, diffuse, moderate to marked with lympho-plasmacytic and
histiocytic choroiditis and phlebitis, Tonkinese cat, Felis
catus.
Conference Comment:
Although there is some moderate slide variability, we thank the contributor for providing an excellent example and review of
feline infectious peritonitis (FIP), a disease that is caused by a mutated
feline enteric coronavirus (mutated FCoV). Given the history provided by the
contributor, this case is likely more consistent with the non-effusive form of
FIP with lesions restricted to the central nervous system, although the
distinction between the wet and dry form is somewhat arbitrary and the disease
likely represents a continuum rather than two distinct clinical forms.3
As mentioned by the contributor, neurologic signs due to encephalitis or
meningitis are present in approximately 60% of all FIP cases.4,5
Chronic ventriculitis, choroiditis, and ependymitis causes outflow obstruction
of the cerebrospinal fluid from the ventricular system of the brain leading to
marked dilation of the ventricles.5 This dilation of the ventricles
leads to a compression atrophy of the proximate neuroparenchyma because of the
lack of expansibility within the skull. The resulting increased intracranial
pressure then results in both hydrocephalus and caudal displacement and
herniation of the cerebellum through the foramen magnum, present in this case.
FIP remains as one of the most common causes of infectious death in cats with
Bengals, Birmans, Himalayans, ragdolls, and Rexes significantly overrepresented
for the development of the disease.3
A recent publication in Veterinary Pathology
by Kiper and Meli1 outlined three key features as prerequisites for
the development of FIP: a) systemic infection with virulent mutated FCoV, b)
effective viral replication in circulating monocytes, and c) activation of
mutated FCoV-infected monocytes.1 Although avirulent FCoV is
readily transmitted via the fecal-oral route, most believe that the mutated
virulent form is not transmitted horizontally, but is rather the result of
spontaneous mutation within each cat that develops FIP. The hallmark lesion of
FIP is granulomatous or lymphohistiocytic phlebitis, present in this case,
which is mediated by activated circulating monocytes during viremia.
Activated monocytes upregulate adhesion molecules
such as CD18, and produce pro-inflammatory cytokines such as TNF-α, IL-1b,
GM-CSF, and IL-6, in addition to matrix metalloproteinases (MMP-9), and
vascular endothelial growth factor (VEGF). Endothelial cells appear to be
selectively responsive and activated by the cytokine storm generated, which
limits the distribution of lesions to veins within select organs. The trigger
for the massive monocyte activation and selectivity of lesion location is not
currently known.1
Conference participants discussed the nature of the immune
response by the host as a determining factor for which form of the disease the
animal will have. As mentioned by the contributor, mutated FCoV-infected
circulating monocytes are likely responsible for viremia. The conference
moderator instructed that cats with a strong cell-mediated immune (CMI)
response do not develop FIP.1,3,5 Alternative, a weak CMI and strong
humoral response results in the effusive, or wet form of the disease. This form
is characterized by vasculitis, peritonitis and profound thoracic and
peritoneal effusion as a result of deposition of antigen-antibody complexes
(type III hypersensitivity).3,5 In addition, these cats are
hypergammaglobulinemic due to the overproduction of ineffective antibodies. In
contrast, the noneffusive dry form of the disease is associated with a moderate
CMI response with pyogranulomatous inflammation (type IV hypersensitivity) and
develop clinical signs based on the organs affected, such as the brain in this
case. However, as noted above, the different forms represent a continuum
and most cases are likely a mix of the two extreme forms of the disease. 1,3,5
References:
1. Kipar A, Meli
ML. Feline infectious peritonitis: still an enigma? Vet Pathol. 2014;
51(2):505-526.
2. Pedersen NC: A
review of feline infectious peritonitis virus infection: 1963-2008. J Feline
Med Surg. 2009; 11(4):225-258.
3. Uzal FA,
Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb,
Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th
ed. Missouri: Elsevier; 2016:253-254.
4. Vandevelde M,
Higgins RJ, Oevermann A. Veterinary Neuro-pathology: Essentials of Theory
and Practice. Oxford: Wiley-Blackwell; 2012.
5. Zachary J,
McGavin M. Nervous system. In: Pathologic Basis of Veterinary Disease. 5th
ed. Missouri: Elsevier; 2012:860-861.

Click the slide to view.
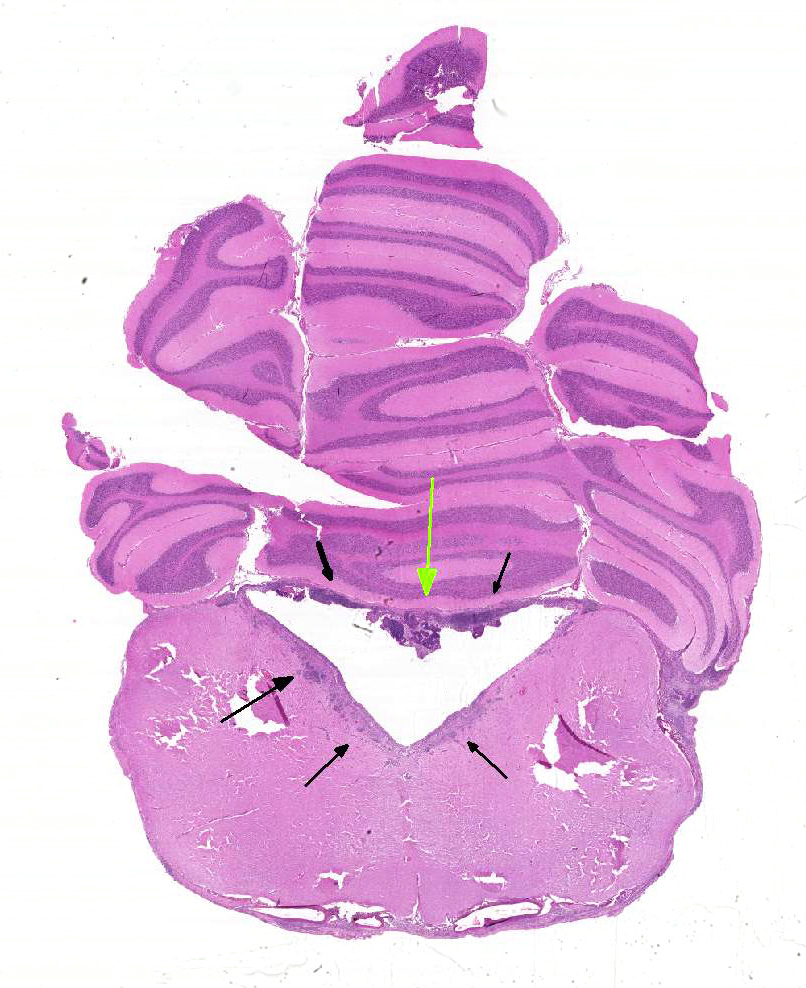
2-1 Cat, fourth ventricle.
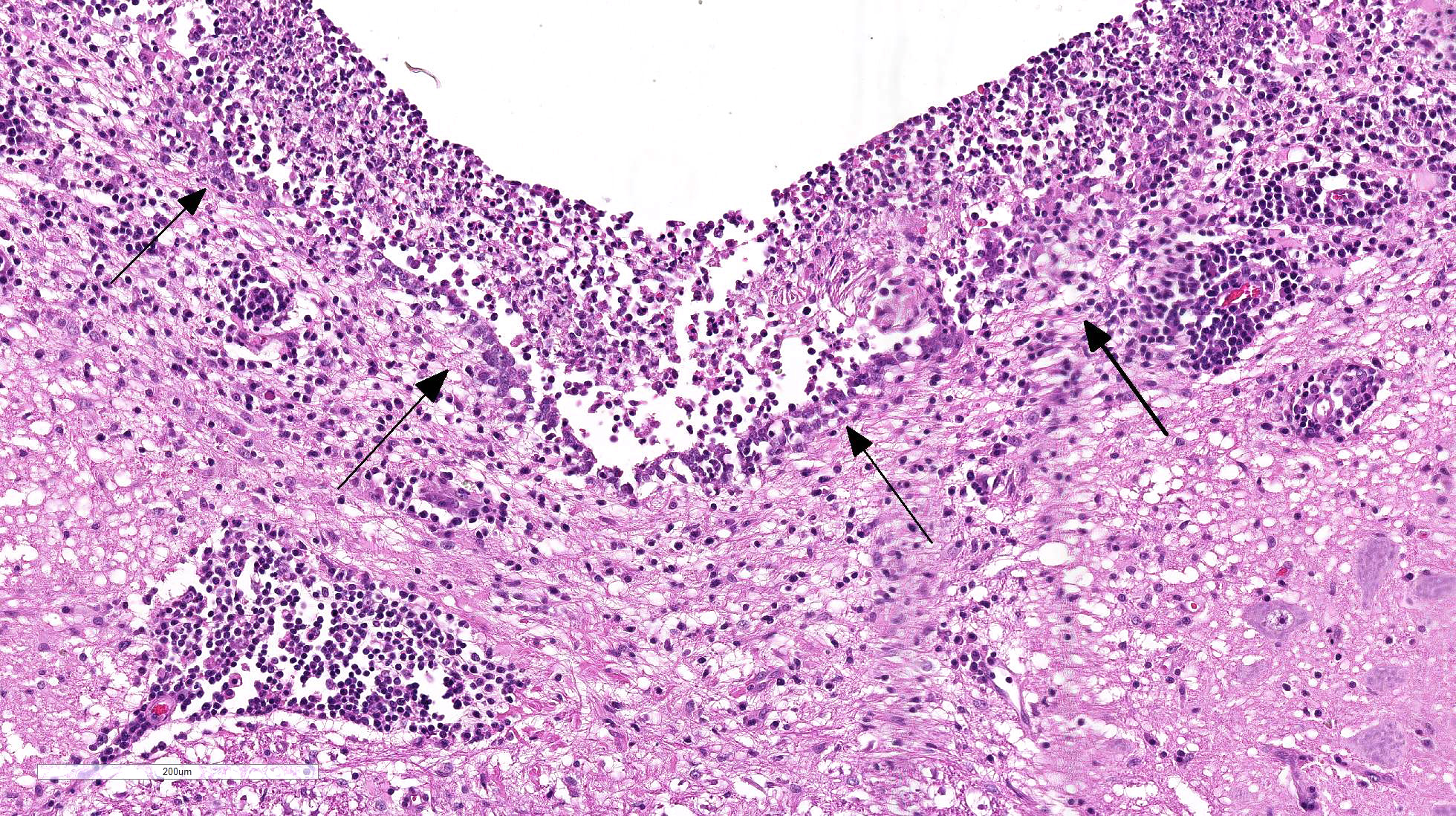
2-2. Cat, fourth ventricle and underlying brainstem.
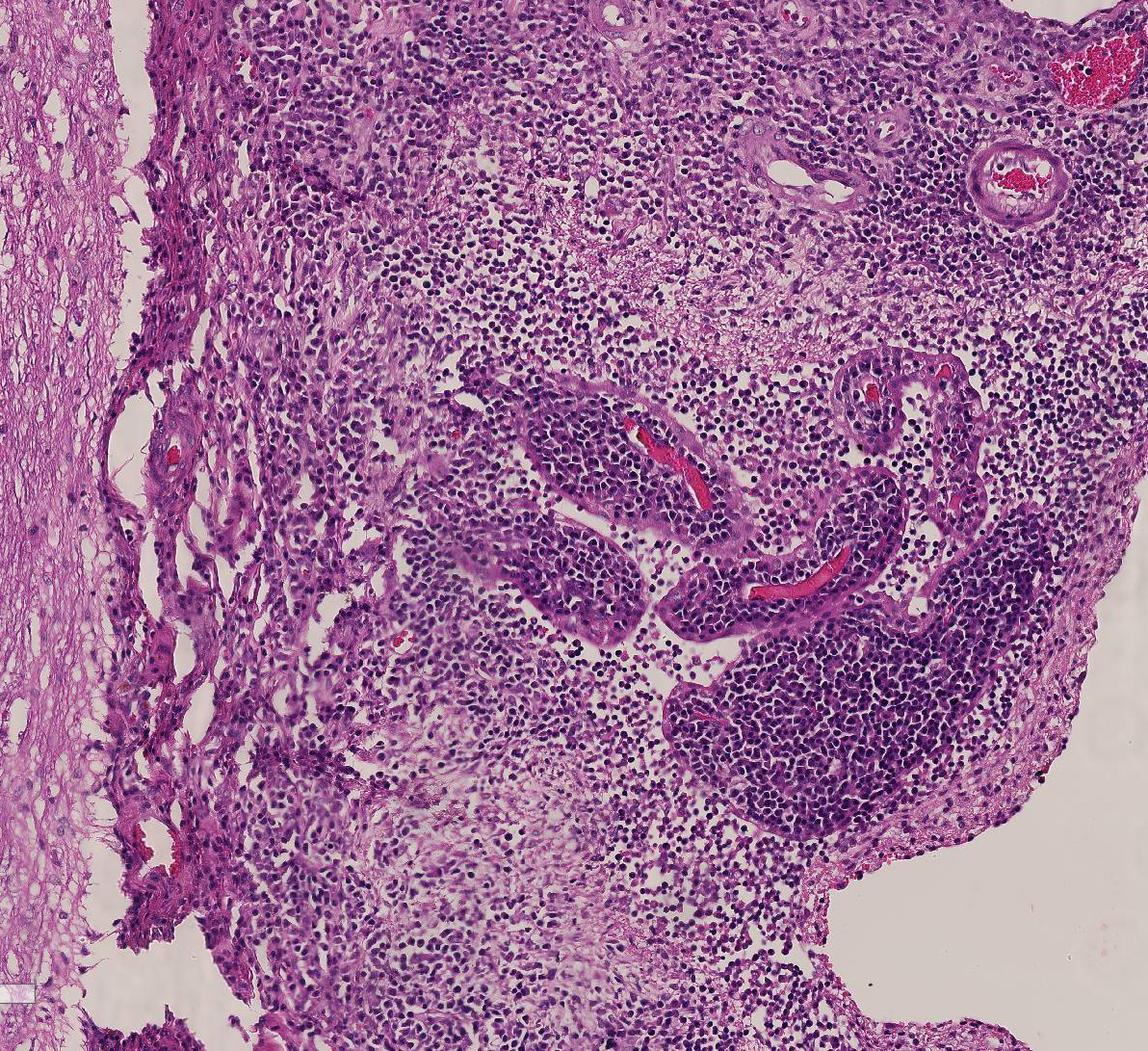
2-3. Cat, brainstem meninges:
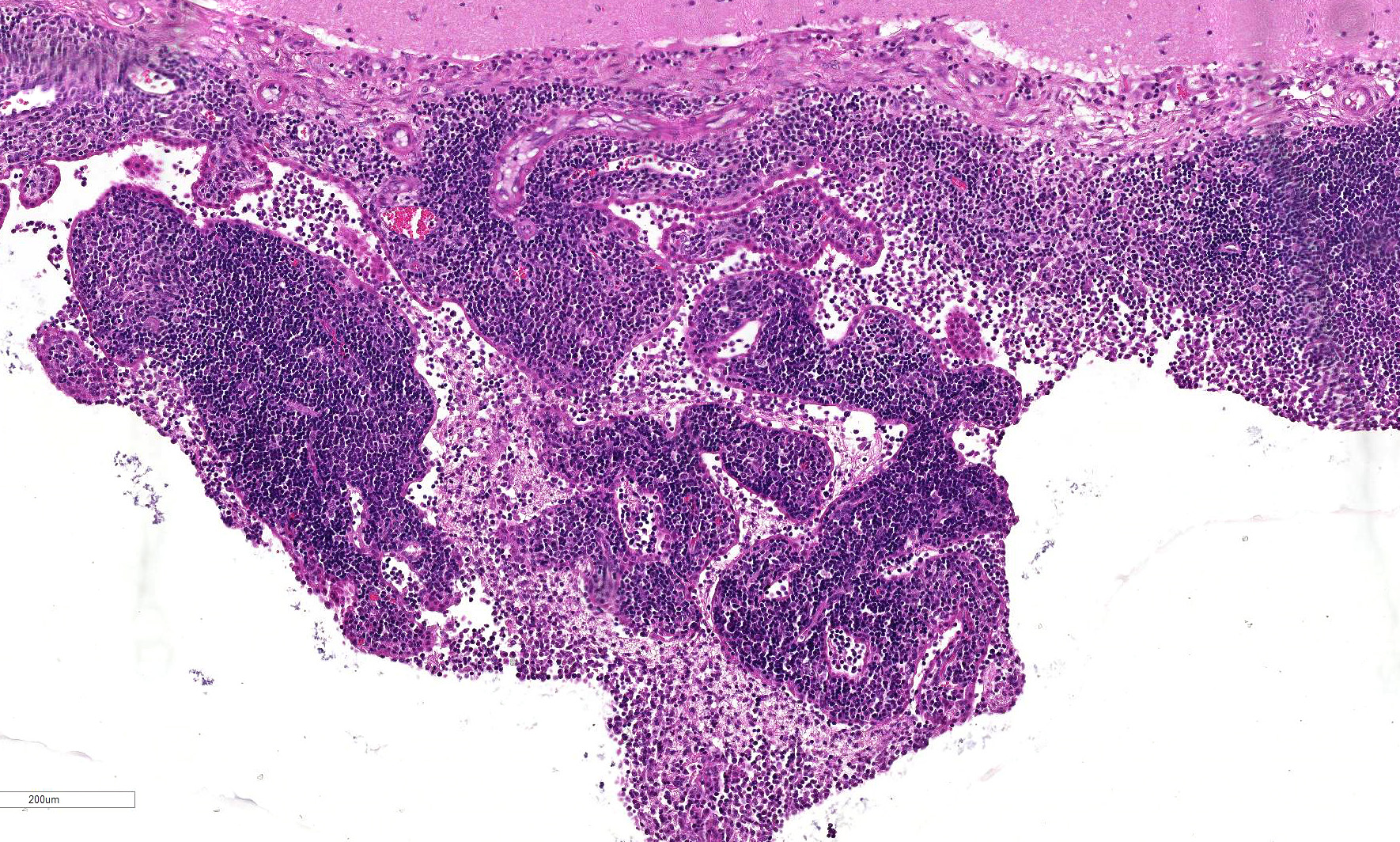
2-4. Cat, choroid plexus, fourth ventricle.
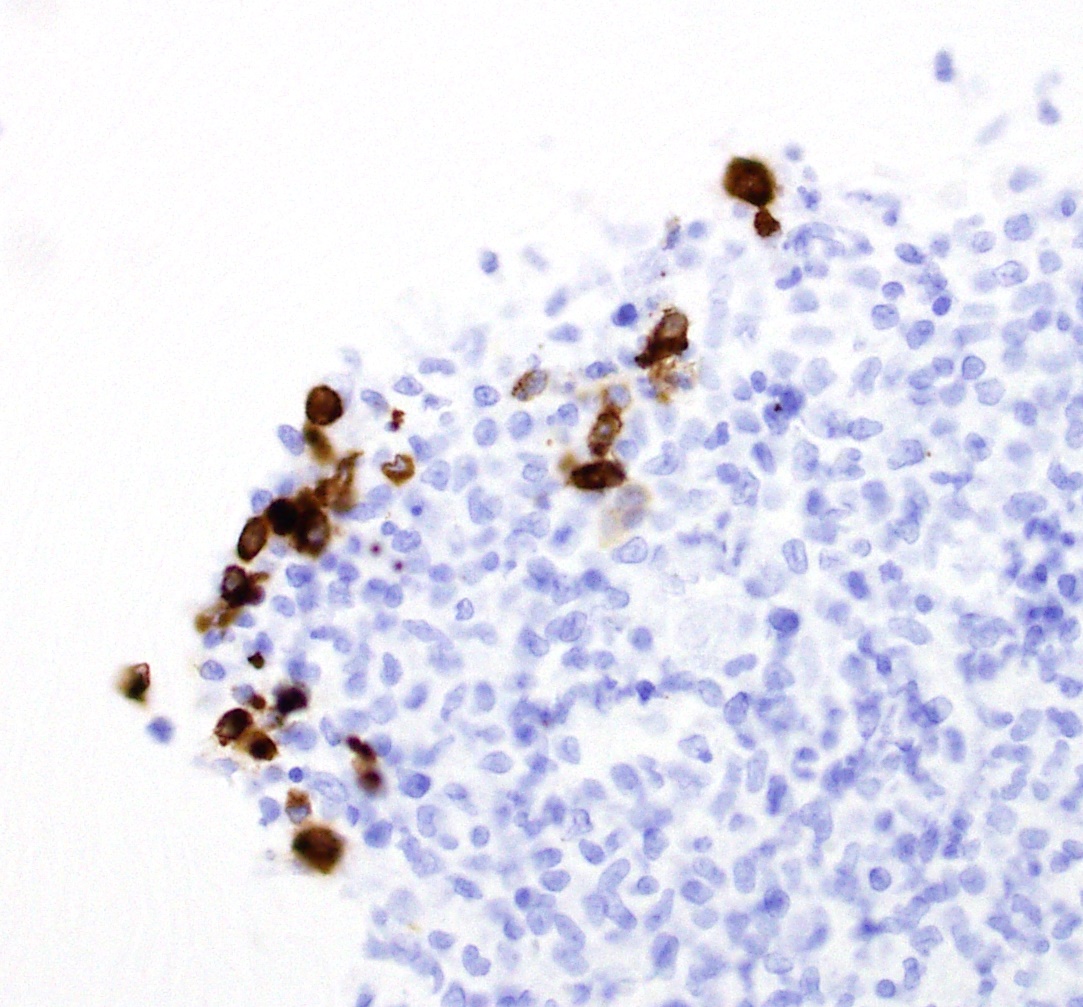
2-5. Cat, fourth ventricle:




