Wednesday Slide Conference, Conference 4, Case 3
Signalment:
7-months-old, male, pit bull terrier, Canis lupus familiaris, canine.
History:
The dog was taken into the animal shelter as a stray. It was found dead in the kennel the next morning.
Gross Pathology:
The dog was in good body condition with mild postmortem decomposition. The lungs were congested, heavy, and wet. The liver and spleen were congested.
Laboratory Results:
The affected blood vessels in the brainstem contained intact and degenerate endothelial cells that were positive for canine adenovirus using immunohistochemistry.
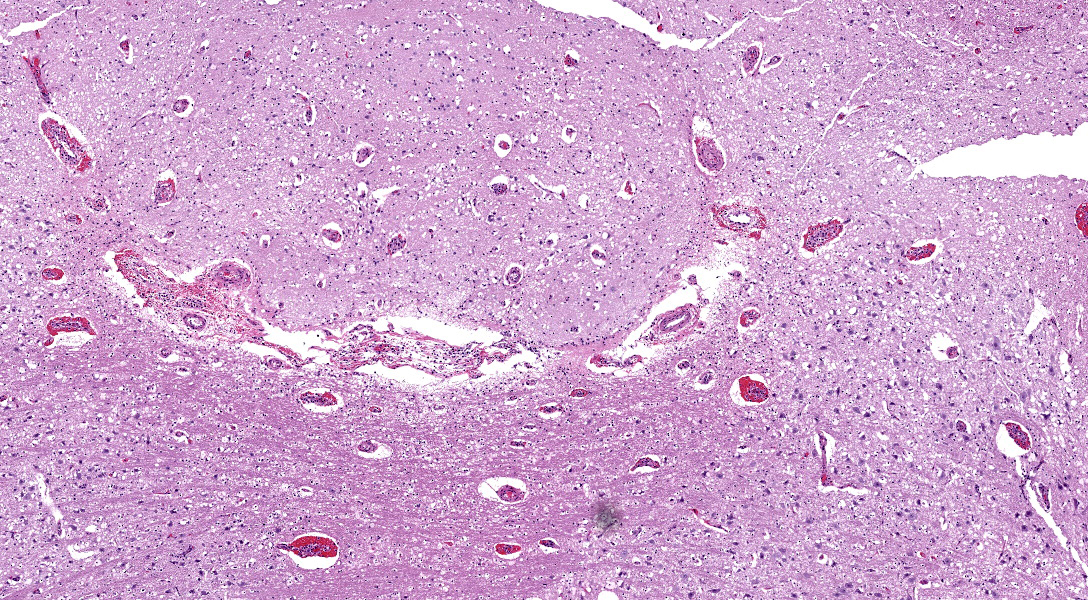
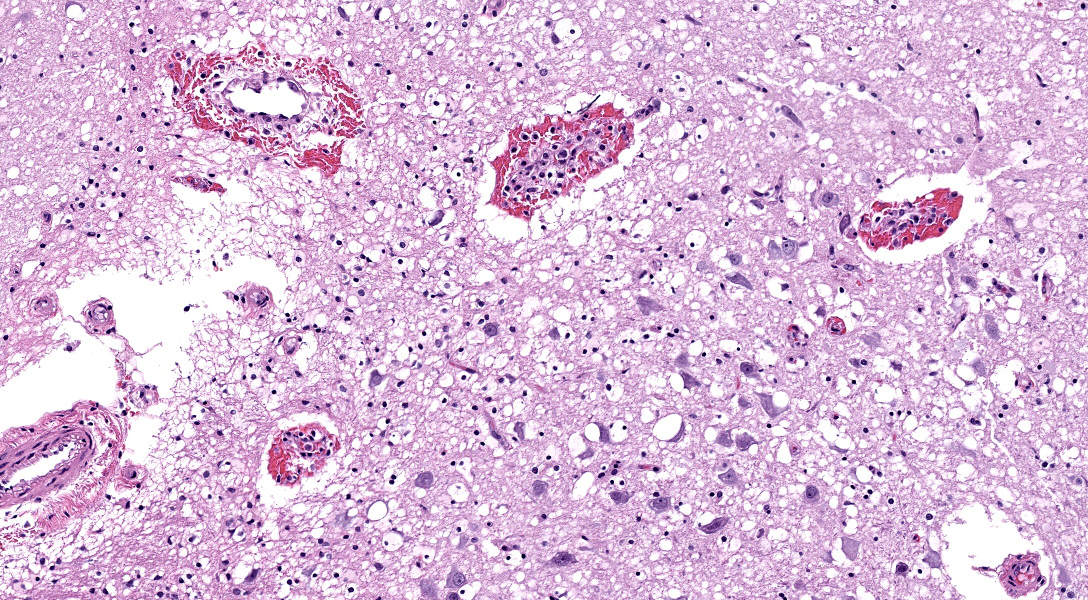
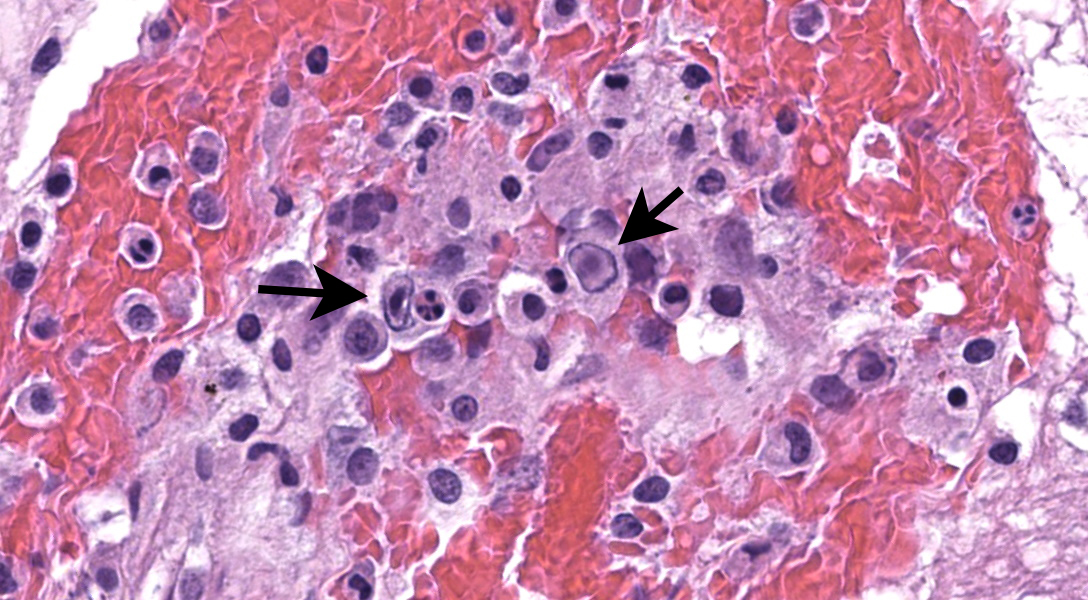
Microscopic Description:
There are multifocal hemorrhages in the neuropil of the brainstem. The capillaries and venules are dilated. The affected capillaries and venules are often lined by swollen endothelial cells and karyorrhectic endothelial cells. Multifocally, there are intranuclear inclusion bodies in the vascular endothelial cells. In a few areas, the tunicae media of the blood vessels are expanded by brightly eosinophilic, homogenous to beaded material admixed with scattered pyknotic and karyorrhectic debris (fibrinoid necrosis) and infiltrated by lymphocytes, and macrophages(vasculitis).
Contributor’s Morphologic Diagnosis:
Brainstem – Vasculitis with endothelial cell necrosis, intranuclear inclusion bodies in endothelial cells, and perivascular hemorrhage; etiology, canine adenovirus - 1
Contributor’s Comment:
Infectious canine hepatitis (ICH) is an uncommon disease of dogs that is caused by canine adenovirus-1 (CAV-1).2-7 CAV-1 is a non-enveloped, icosahedral, double-stranded DNA virus.6,7 CAV-1 can infect and cause disease in domestic dogs, wild canids, skunks, and bears.2-7 In domestic dogs, ICH is uncommon due to the routine vaccination of dogs, but ICH can be seen in unvaccinated dogs.2-7 ICH is typically seen in young dogs less than 1 to 2-years-old, but any dog not vaccinated for canine adenovirus can develop ICH. CAV-1 is antigenically related to canine adenovirus-2, which causes respiratory disease in dogs.
CAV-1 is secreted in the saliva, urine, and feces of infected dogs.2,-7 Transmission to a naïve dog is by oronasal exposure from dog-to-dog contact or from the contaminated environment. After oronasal exposure, CAV-1 infects the tonsils causing tonsillitis. The virus will then spread to the regional lymph nodes and then to the blood causing viremia 4 – 9 days after exposure, which corresponds to the incubation period of ICH. Viremia of CAV-1 results in dissemination to the hepatocytes, endothelial cells, and mesothelial cells. Disease caused by CAV-1 can be divided into three syndromes.4,6,7 Peracute disease that occurs within a brief illness that ranges from 3 – 48 hours and is characterized by circulatory collapse and death. Acute ICH is characterized by fever, depression, anorexia, vomiting, diarrhea, petechiae on the mucus membranes, pale mucus membranes, and mild icterus. Acute ICH can be fatal. The last syndrome of ICH is a mild chronic disease where the infected dog has partially immunity and may recover or die weeks to months later due to chronic liver disease.
CAV-1 infection of its target cells corresponds to the lesions seen grossly.2-7 There can be enlargement and reddening of the tonsils and lymph nodes in the area. Petechiae on serosal surfaces and clear fluid in the peritoneal and surfaces can occur. The liver can be swollen, turgid, and friable with fine yellow mottling to a distinct accentuated reticular pattern. There can be fibrin on the surface of the liver. The gallbladder can be variably edematous, and gallbladder edema is considered to be pathognomonic of ICH. Icterus is mild when present. There can be hemorrhagic infarcts in the renal cortex as well as hemorrhages in the lungs. Hemorrhage and necrosis can occur at the metaphysis of long bones. The brainstem and midbrain can have hemorrhages in a small percentage of cases.1-7 The late development of corneal edema due to a type III hypersensitivity reaction corresponding to increasing neutralizing antibodies can occur with ICH.2,3,4,6,7
The microscopic lesions of ICH in the liver are of centrilobular necrosis with intranuclear inclusion bodies in hepatocytes adjacent to the necrotic foci.2-7 The necrotic foci contain small numbers of infiltrating leukocytes mainly neutrophils heterophils. The microscopic lesions in the other organs are the result of the endothelial cell injury secondary to infection with CAV-1. CAV-1 infection of endothelial cells in the renal glomeruli can cause glomerulonephritis, and infection of renal tubules cells can result in virus shedding in the urine. Pulmonary hemorrhages are secondary to vascular damage in the lung. Corneal edema is secondary to the immune response to CAV-1 infection of the corneal endothelium. When brain lesions due occur, there tend to occur in the brainstem and midbrain and consist of hemorrhages secondary to endothelial damage.1,-7 Widespread endothelial damage can result in disseminated intravascular coagulopathy, which can also result in widespread petechiae in serosal surface and multiple organs and death of the dog.2-7
Contributing Institution:
New Mexico Department of Agriculture Veterinary Diagnostic Services
https://nmdeptag.nmsu.edu/labs/veterinary-diagnostic-services.html
JPC Diagnosis:
Pons: Vasculitis, necrotizing, acute, diffuse, moderate, with rare neuronal necrosis, mild gliosis, and endothelial intranuclear viral inclusions.
JPC Comment:
The contributor provides a succinct summary of CAV-1 and ICH. Connecting the dots to the present case, this section features diffuse vascular mural and transmural hemorrhage and edema attributable to endothelial intranuclear adenoviral inclusions. IHC for adenovirus performed by the contributor strongly and specifically labeled these endothelial cells, confirming the diagnosis. Conference participants felt that there was also microglial activation resulting from early parenchymal inflammation . These microglial cells were nicely highlighted by IBA1 (ionized calcium-binding adapter molecule 1) immunostain, which highlights dendritic cells, macrophages, and in the neuroparenchyma, microglia. Early characterization of this protein was performed in rat brain microglia.9 In our experience, IBA1 has generally worked well across species and expression seems to be reasonably conserved.
Neurologic manifestation of CAV-1 is rare in domestic canines1,5; the animal in this case was reportedly a stray with an unknown vaccination history.
References:
- Caudell D, Confer AW, Fulton RW, Better A, Saliki JT, Fent GM, Ritchey JR. Diagnosis of infectious canine hepatitis virus (CAV-1) in puppies with encephalopathy. J Vet Diagn Invest. 2005;17:58-61.
- Cullen JM and Stalker MJ. Liver and biliary system. In: Maxie GM, ed. Jubb Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. vol 2. Elsevier; 2016.
- De Jonge B, Van Brantegem L, Chiers K. Infectious canine hepatitis, not only in the textbook: a brief review and three case reports. Vlaams Diergeneeskd Tijdschr. 2020;89: 284-291.
- Green CE. Infectious canine hepatitis and canine acidophil cell hepatitis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Elsevier; 2012.
- Hornsey SJ, Philibert H, Godson DL, Snead ECR. Canine adenovirus type 1 causing neurological signs in a 5-week-old puppy. BMC Vet Res. 2019 Nov 21;15(1):418.
- Knowles DP. Adenoviridae. In: MacLachlan NJ and Dubovi EJ, eds. Fenner’s Veterinary Virology. 4th ed. Elsevier; 2011.
- Sykes JE. Infectious canine hepatitis. In: Sykes JE, ed. Canine and Feline Infectious Diseases. 1st ed. Elsevier; 2014.
- Van Wettere AJ and Brown DL. Hepatobiliary system and exocrine pancreas. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. Elsevier; 2022.
- Ito D, Imai Y, Ohsawa K, et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research 1998; 57(1): 1-9.