Signalment:
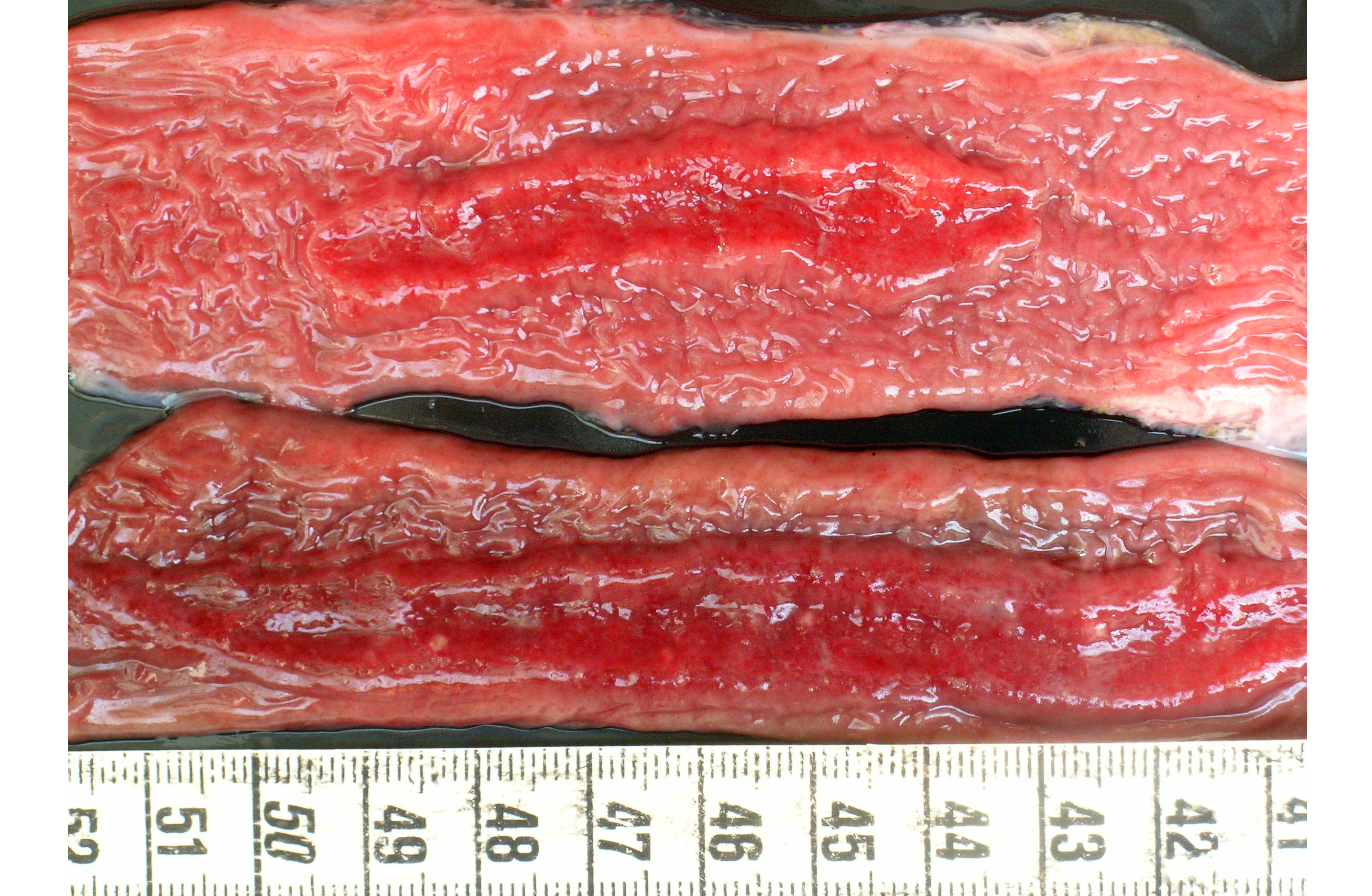
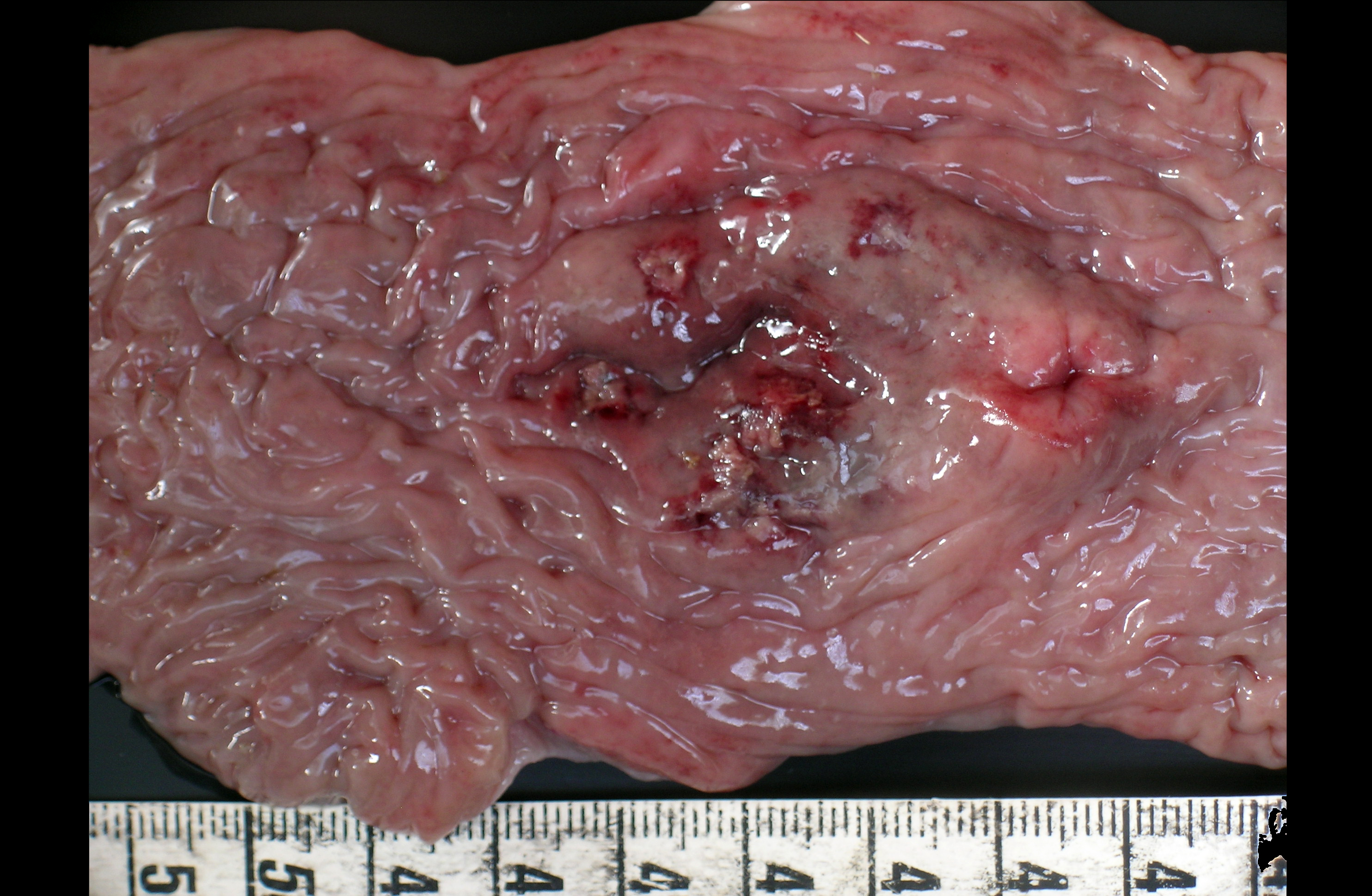
Gross Description:
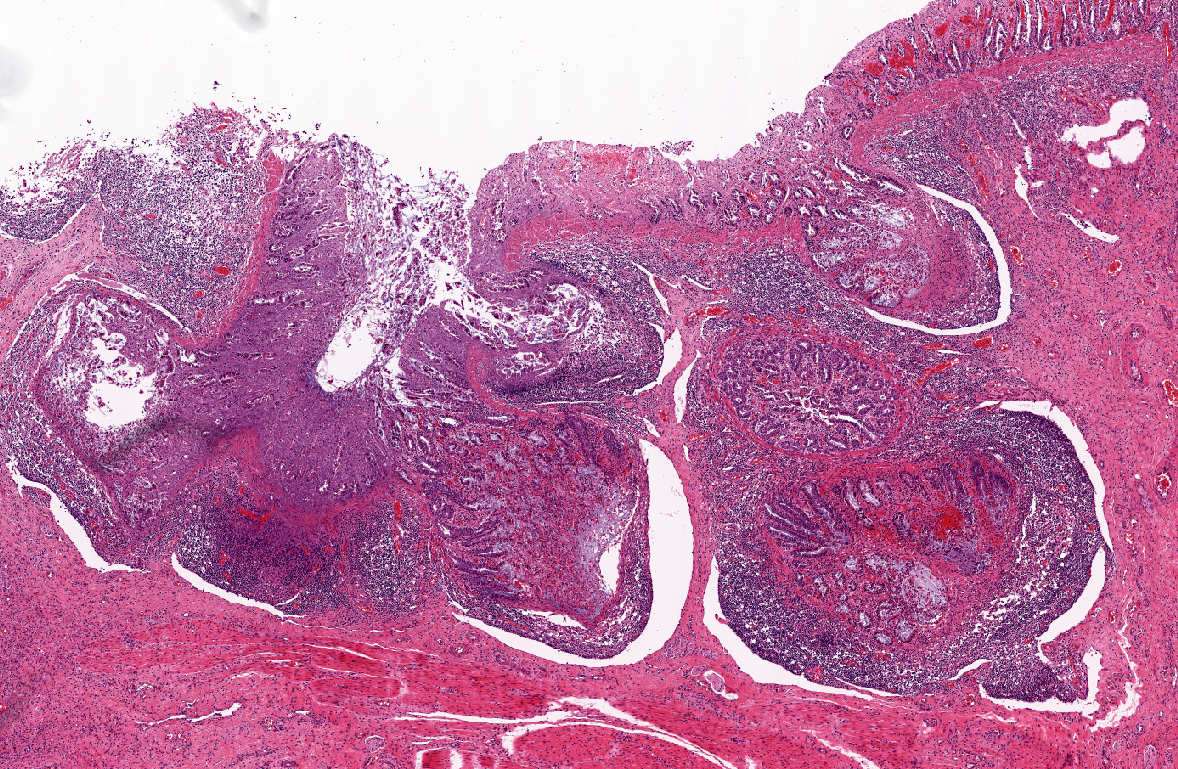
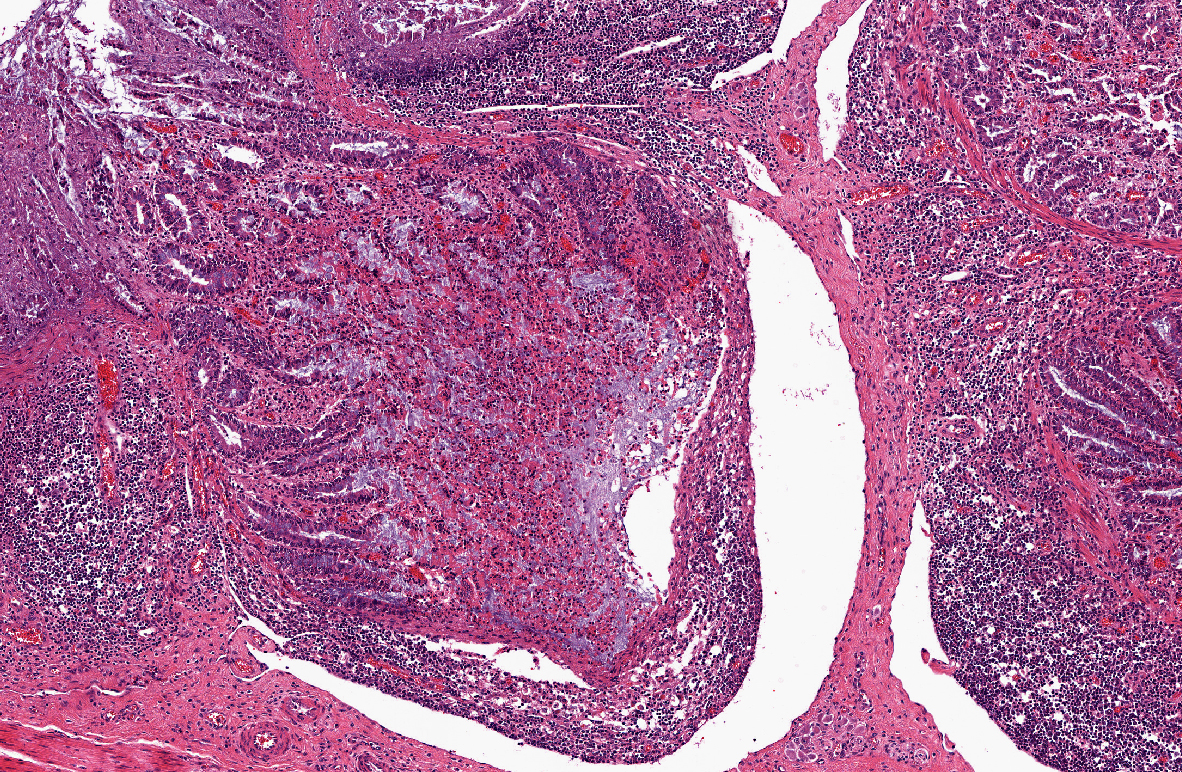
Histopathologic Description:
Morphologic Diagnosis:
1) Enterocolitis, multifocal, necrotizing and fibrino-hemorrhagic, severe, acute with severe Peyers patch necrosis and intranuclear inclusion bodies (adenoviral type).Â
2) Colitis, multifocal, submucosal, neutrophilic, chronic moderate with granulation tissue formation and intralesional bacterial colonies.
Lab Results:
Condition:
Contributor Comment:
Microscopic lesions were found in the respiratory (bronchiolitis and bronchopneumonia) and intestinal tract (necrosis, fibrinous exudates, hemorrhage, mild infiltration with mononuclear cells and granulocytes) whereas the basophilic or amphophilic vascular inclusion bodies were identified in the lung, kidney, liver, abomasum, small intestine and colon(2,4). Necrosis preferentially affected Peyers patches with extension to the overlying mucosa. In the described cases the pathologic findings were related to the primary vascular damage produced by the virus, supported by the absence of other enteric pathogens(3,4,5). Bacteria, when present, are considered secondary opportunists, but potential contributors to the ultimate pathologic process(3). The vessels sometimes contained thrombi with many leukocytes and neutrophils present in the walls(3). However further experimental studies are needed to determine the virulence of BAdV and to establish if its presence in the vascular endothelium is a cause or a consequence of a co-existing enteric disease(5).
In other domestic species like horses and pigs, enteric adenovirus infection targets the epithelial cells, yielding to villus atrophy, epithelial sloughing and mild diarrhea. This differs from what seen in calves in which the intestinal pathology is associated with primary infection of blood vessels resulting in necrosis of the mucosa and lymphoid tissue.
The disease must be differentiated from common causes of fibrinous and hemorrhagic enterocolitis, including coccidiosis, bovine viral diarrhea, salmonellosis and bovine malignant catarrhal fever. There is very limited information available on the prevalence of BAdVs, particularly BAdV 10 infection within cattle populations(5). At present, adenoviral infection of cattle with enteric disease is probably underdiagnosed especially because there is no test suitable for diagnosis of the disease in live animals and, second, because diagnostics require histopathologic or immunocytochemical examination of the intestine(4, 5). It has been speculated that the sporadic cases of fatal BAdV 10 infection can be the result of some type of immunological incompetence(2). Further confirmation through electron microscopy, in situ hybridization or virus isolation in tissue cell cultures was not possible at that time.
JPC Diagnosis:
Conference Comment:
The use of the term crypt abscess to describe the material filling the crypts is considered by some to be a misnomer as the material is often composed of necrotic epithelial and inflammatory cells within a crypt lumen, rather than suppurative inflammation of the crypt epithelium. The moderator prefers cryptitis as a more descriptively accurate term for the lesions in this case. Crypt abscess would more appropriately describe the lesions associated with ulcerative colitis where a suppurative inflammatory process of the epithelium predominates; however, the term crypt abscess is widely used by veterinary pathologists and is generally understood.
References:
2. Lehmkuhl HD, Cutlip RC, DeBey BM: Isolation of a bovine adenovirus serotype 10 from a calf in the United States.J Vet Diagn Invest 11: 485-490, 1999
3. Orr JP: Necrotizing Enteritis in a calf infected with adenovirus. Can Vet J 25: 72-74, 1984
4. Smyth JA, Benk+�-� M, Moffett DA, Harrach B: Bovine Adenovirus Type 10 identified in fatal cases of adenovirus-associated enteric disease in cattle by in situ hybridization. J Clin Microbiol 34: 1270-1274, 1996
5. Smyth JA, Moffett DA, Garderen E, Orr JP: Examination of adenovirus-types in intestinal vascular endothelial inclusions in fatal cases of enteric disease in cattle, by in situ hybridization. Vet Microbiol 70: 1-6, 1999
6. Thompson KG, Thomson GW, Henry JN: Alimentary tract manifestations of bovine adenovirus infections. Can Vet J 22: 68-71, 1981
7. Ursu K, Harrach B, Matiz K, Benk+�-� M: DNA sequencing and analysis of the right-hand part of the genome of the unique bovine adenovirus type 10. J Gen Virol 85: 593-601, 2004