Signalment:
17-year-old, female, French trotter horse (
Equus caballus). A 17-year-old female horse was referred to the Veterinary School of Nantes for clinical signs of respiratory difficulty and persistent left sinus deformation.
Physical examination revealed a bilateral deformation of gums with multifocal ulceration. RMI showed maxillary bone and turbinate replacement by a material of fibrous density. The horse was euthanized for humane reasons and a complete necropsy examination was performed shortly after death.
Gross Description:
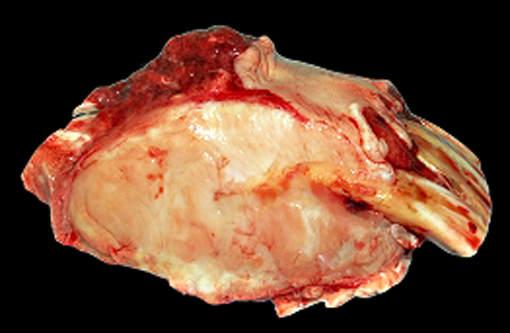
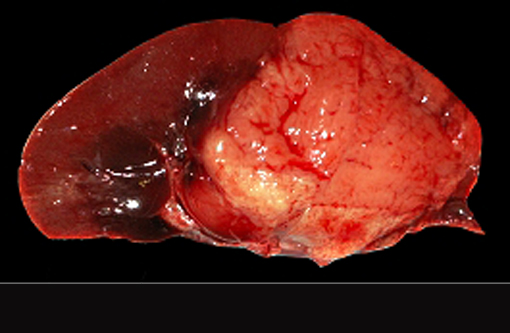
At necropsy, a severe bilateral rostrocaudal osteolysis of maxillary bone was observed. Bone was replaced by a white, firm, fibrous tissue. Other macroscopic findings include a 6 cm diameter, white, firm, homogenous, and well-delimited nodule on the left kidney.
Histopathologic Description:
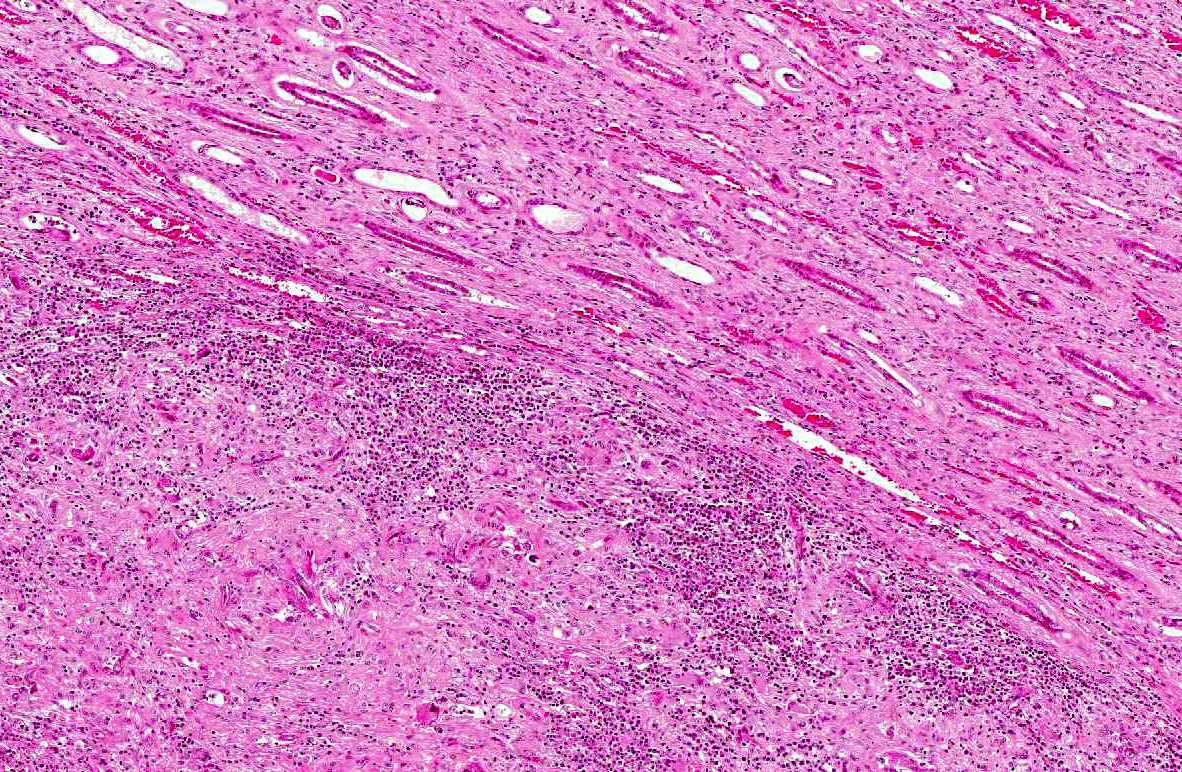
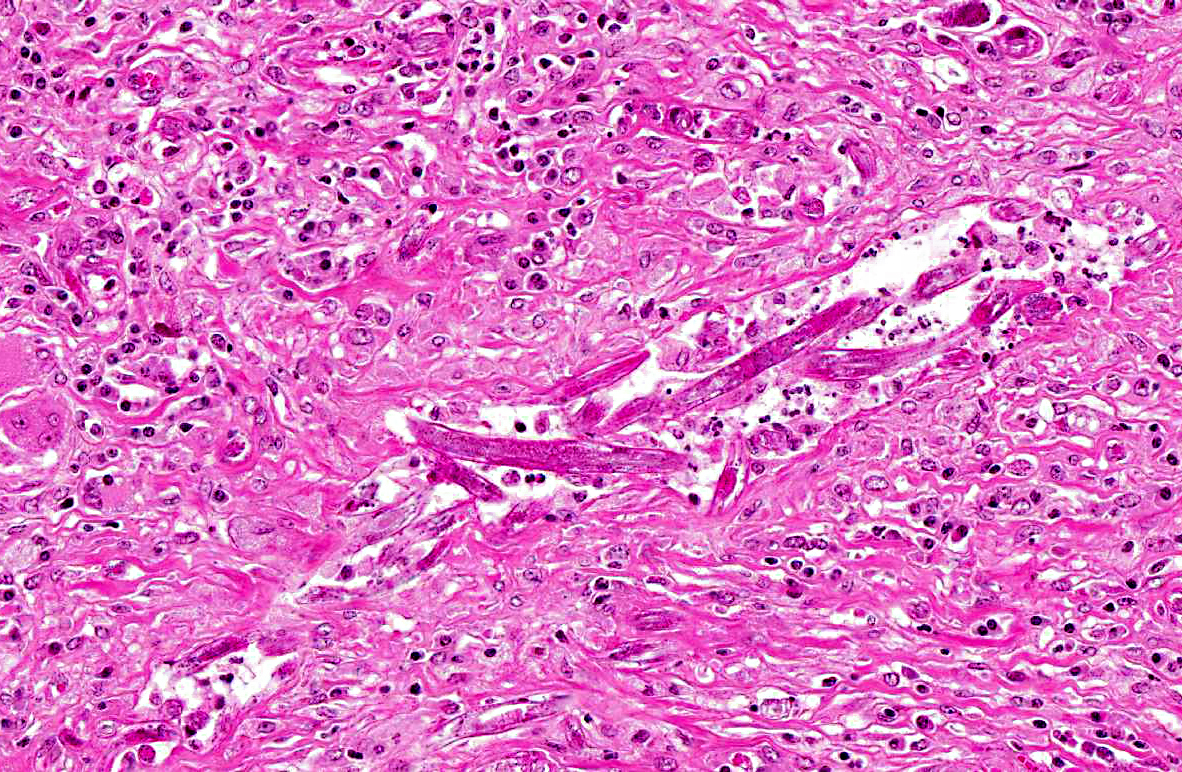
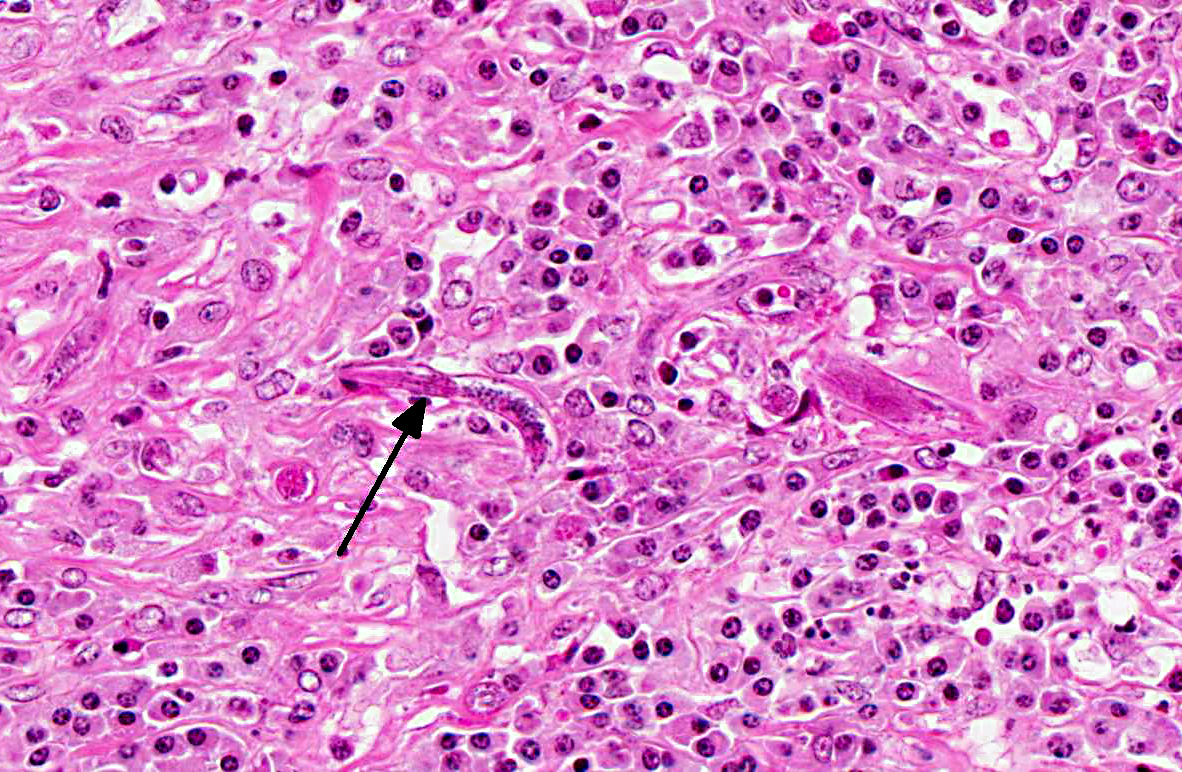
The renal architecture was disrupted by multifocal coalescent granulomas cantered on numerous tangential and cross-sections of well-preserved round worms (nematodes). The inflammatory infiltrate mainly consisted of epithelioid cells, multinucleated giant cells, numerous plasma cells, eosinophils and few lymphocytes surrounded by abundant fibrous tissue. Multifocally, foci of dystrophic calcification were adjacent to degenerated nematodes. The nematodes were 250-300 μm length and 15-20 μm diameter, with a smooth thin cuticle, a tapered tail, a uterus containing a dorsoflexed ovary and a long characteristic rhabditiform esophagus with a corpus, an isthmus and a bulb. Smaller nematodes with a length of 150-180 μm and a diameter of 10-15 μm with a rhabditiform esophagus and granular internal basophilic structures were also detected. The nematodes were interpreted respectively as adult and larval stages of
Halicephalobus gingival is (formerly
H. deletrix, Micronema deletrix). Numerous eggs with a length of 35 μm and a diameter 20 μm were also observed.
Morphologic Diagnosis:
Kidney: nephritis, granulomatous, multifocal, coalescent, with
Halicephalobus gingivalis.
Condition:
Halicephalobus gingivalis
Contributor Comment:
H. gingivalis (formerly
Micronema deletrix) is a free-living, saprophytic and opportunistic small nematode of the order
Rhabditida, family
Rhabditidae. Most species inhabit decaying organic matter and are common in soil, foul water and manure.Â
H. gingivalis infections occur sporadically. This parasitic infection is described at least in 60 cases in horses mainly in the USA, Canada, Japan, in few cases in ponies and humans.(1) One case in a horse was recently described in Switzerland but no case has been described in France before. The pathogenesis, life cycle and route of infection of
H. gingivalis are poorly understood.(6) Free-living forms of the parasite in the soil are suspected to infect horses via skin or mucous membrane wounds in recumbent animals, and then disseminate hematogenously or to migrate along blood vessels or nerves to internal parenchymal tissues including the central nervous system.(3,6) Other routes of infection have been suggested, such as: infections from inhalation of the nematodes, possible colostral transfer to foals during nursing (as described in one report involving a Thoroughbred mare with mastitis and a nursing foal with encephalitis).(3,6) Additionally, the possibility of urogenital route has been considered, as viable organisms were noted in the sperm and urine of two stallions.(5)
H. gingivalis life cycle is unknown. In tissues, only adult female, larvae and eggs are observed and asexual reproduction by parthenogenesis is suspected.(5) Free male and female worms are found in soil and a sexual reproduction is suspected. The classical granulomatous lesions of
Halicephalobus in horses are mainly found in the brain, kidneys, oral and nasal cavities, lymph nodes, and spinal cord.(1,3,5) They are also frequently found in the adrenal gland and lung. Infrequent sites of granuloma formation include stomach, bone (mandible, femur, maxilla, nasal bones), ganglia, heart, liver, and eye, testicle and prepuce.(5,6) In human cases, granulomatous lesions are always found in brain and sometimes in the heart and liver.Â
The other rhabditid parasites infecting the horse are:
Pelodera strongyloides, Strongyloides westerii and
Cephalobus sp. Pelodera strongyloides causes a self-limiting dermatitis normally confined to the ventral abdomen.Â
Pelodera is mostly observed in dogs and rarely in sheep.Â
S. westerii larvae causes a cutaneous infection but adults and eggs are not found in the skin. In cutaneous and mucocutaneous lesions
Cephalobus can be distinguished from
H. gingivalis by its esophagus and stoma shape and blunt posterior end.(5,6)
JPC Diagnosis:
Nephritis, granulomatous and eosinophilic, focally extensive, severe with adult and larval rhabditoid nematodes.
Conference Comment:
The contributor provided a very good description and discussion of the classic lesions caused by
Halicephalobus gingival is in horses. Conference participants, while discussing the pathogenesis of this disease, elaborated on the role of eosinophils in parasitic infections and reviewed several aspects of eosinophilic inflammation. Eosinophilia (defined as an increase in the number of eosinophils in tissue or blood) is generally observed in parasitic disease, hypersensitivity, and in some neoplasms. Eosinophils can be recruited in cases of both ectoparasitism and endoparasitism. Additionally, they play a role in the late phase of type I (immediate) hypersensitivity reactions (anaphylaxis, allergies, asthma etc.). Neoplastic diseases that often recruit eosinophils include eosinophilic leukemia, mast cell tumor, T-cell lymphoma, lymphomatoid granulomatosis, various carcinomas, fibrosarcoma, and thymoma.(4) Eosinophils are produced and mature in bone marrow. IL-5 is the most important cytokine involved in eosinophil production, influencing eosinophil proliferation, differentiation and maturation, as well as function. GM-CSF and IL-3 also play more minor roles in eosinophilic development.(4)
Eosinophils contain distinct granules, called secondary (aka specific) granules, which are lysosomes that contain several components, including major basic protein, acid hydrolases, eosinophilic cationic protein, and eosinophil-specific peroxidase.(2,4) Although eosinophils are also capable of phagocytizing and killing bacteria, they are not as adept at this as neutrophils, and generally cannot clear a bacterial infection independently. Their main function is against large organisms (such as helminths) that are too large to be phagocytized.(4) Under the influence of a Th2 biased immune response in a helminth-infected host, eosinophils are recruited predominantly by IL-5, as well as IL-2 and IL-16. These cytokines induce mature eosinophils to emigrate from the blood and respond to chemoattractants (such as eotaxin, aka CCL-11) to home in to the helminth-infected tissue. There eosinophils bind to immunoglobulins (particularly IgE) and complement components (C1q, C3b/C4b, iC3b, C5a) on the surface of their targets. They may also bind to ligands on the parasite surface (Lewis-related molecules and cell-adhesion molecules). Once activated by antigen-IgE complexes, eosinophils secrete cytokines and chemokines (IL-2, IL-4, IL-10, IL-12, IL-16, IL-3, IL-5, eotaxin, TGF-alpha, VEGF, TNG-alpha, among others) and proinflammatory mediators (substance P).(2) Additionally, they degranulate to release their cytotoxic substances with the following effects: Major basic protein is toxic to helminths, as well as tumor and host cells; it also activates platelets, neutrophils mast cells and basophils. Eosinophilic cationic protein is also toxic to helminths and host cells. Eosinophilic specific peroxidases generate toxic oxygen radicals, providing a further weapon against helminths. Acid hydrolases have digestive functions. Once they degranulate, eosinophils die via apoptosis.(2) Although studies have shown that there may be species variability in the significance of the impact of IL-5 and eosinophils in helminth infections, generally it is accepted that IL-5-induced eosinophilia often plays an important role in the immune response to parasites.Â
References:
1. Akagami M, Shibahara T, Yoshiga T, et al. Granulomatous nephritis and meningoencephalomyeliis caused by
Halicephalobus gingivalis in a Pony Gelding.
J Vet Med Sci. 2007;69(11):1187-1190.
2. Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice.
Parasitol Today. 2000:16(5);202-209.Â
3. Bryant UK, Lyons ET, Fairfield TB, et al,
Halicephalobus gingivalis-associated meningoencephalitis in Throroughbred foal.Â
J Vet Diagn Invest. 2006;18:612-615.
4. Webb JL, Latimer KS. Leukocytes. In: Latimer KS, ed.Â
Duncan & Prasses Veterinary Laboratory Medicine: Clinical Pathology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2011:54, 74-75.
5. Johnson JS, Hibler CP, Tillotson KM et al. Radiculomeningomyelitis due to
Halicephalobus gingivalis in a horse.Â
Vet Pathol 2001;38:559-561.
6. Muller S, Gryzbowskit M, Sager H, et al. A nodular granulomatous posthitis caused by
Halicephalobus sp. in a horse. Journal compilation. ESVD and ACVD: 2007;19:44-48.