Signalment:
Five-year-old male rhesus
macaque (
Macaca mulatta).This animal was assigned to the
research project of alcohol, HIV infection &
host defense. Ethanol was administered via
the gastric catheter with 30% ethanol in
water (w/v) as a 0.5-hour prime, followed
immediately by a 4.5-hour maintenance
infusion. The concentration of ethanol in
blood was 50 to 60 mM. The animals
received ethanol four consecutive days per
week for the duration of the study. Three
months after ethanol administration, this
animal was intravenously inoculated with
simian immunodeficiency virus (SIV) 251
about one year before sacrifice.
Streptococcus
pneumoniae was inoculated in
right lung seven months before sacrifice.
Six months after SIV inoculation, this
animal began to show chronic, mild
leukocytosis, mild neutrophilia, and
moderate thrombocytopenia. The animal
developed weight loss, loss of muscle mass,
enlargement and restriction of stifles,
enlarged lymphoid tissue, and mild
splenomegaly and hepatomegaly.
Gross Description:
Presented was a severely
thin animal with no body fat. A catheter tube
was inserted on the left dorsal-lateral back,
tunneled through subcutaneous, and ended at
the left side of stomach. The spleen was
markedly enlarged with prominent white
pulp. All peripheral lymph nodes were four
to six times enlarged. This animal had mild
to moderate thymic and muscular atrophy.
Histopathologic Description:
Kidney:
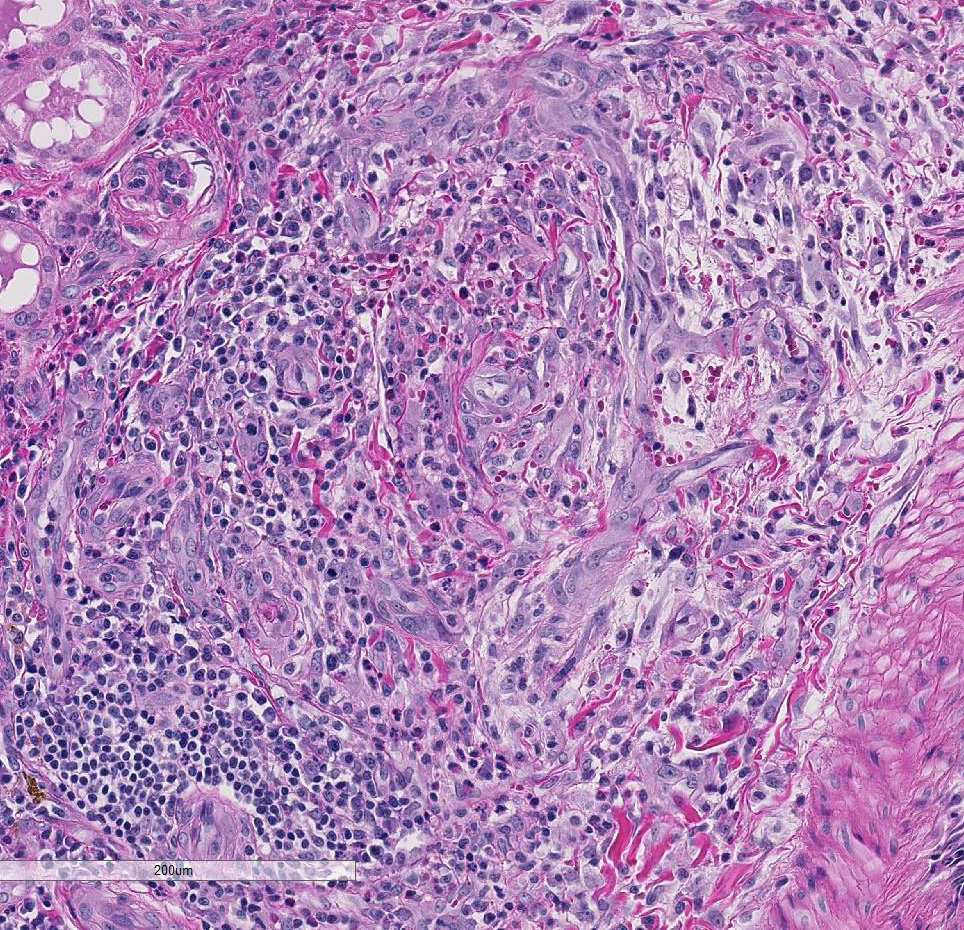
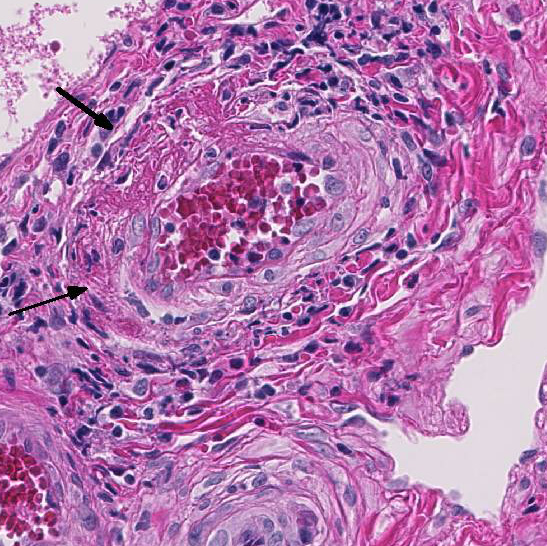
Multifocally the small and medium-sized
arteries are expanded and variably disrupted
by proliferation of the tunica intima, smooth
muscular hyperplasia, and infiltration of
inflammatory cells. The lumina of affected
arteries are partially to completely occluded
and lined by hypertrophic endothelial cells.
Usually, the tunica media are segmentally to
circumferentially thickened by smooth
muscle hyperplasia, fragmented collagen
bundles, and reactive fibroblasts. The tunica
adventitia is markedly expanded by numerous
neutrophils, lymphocytes, plasma
cells, and fewer macrophages and
eosinophils. Some subendothelial tunica
intima and tunica media are disrupted and
markedly expanded by thick bands of deeply
eosinophilic hyaline to fibrinoid material
admixed with cellular and karyorrhectic
debris and many erythrocytes (necrosis and
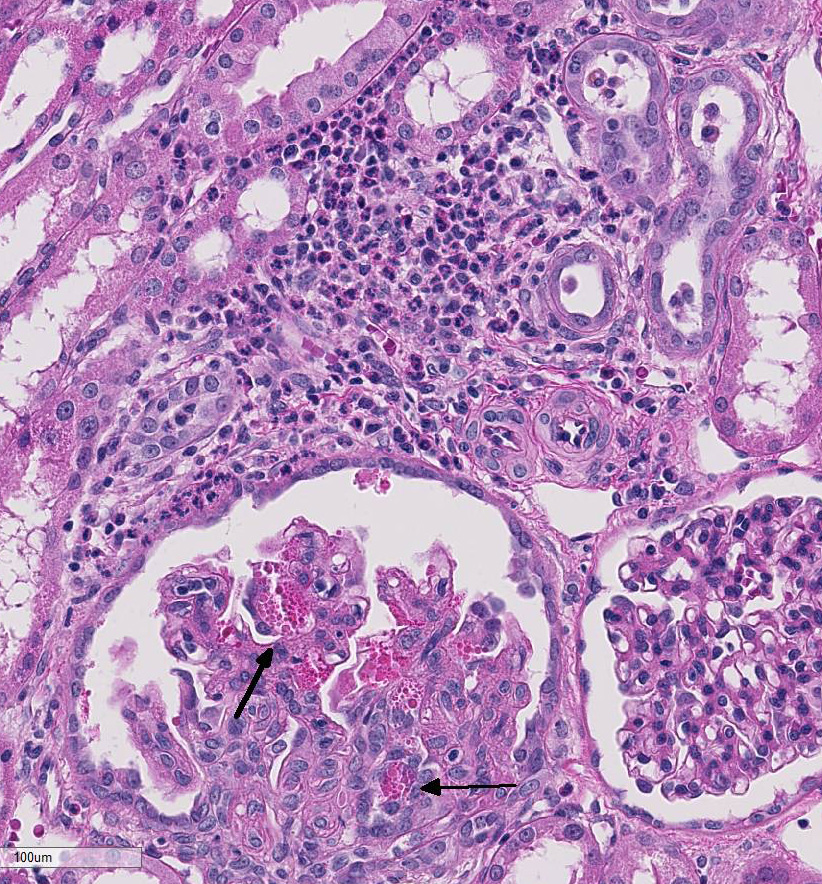
hemorrhage). Multifocally the renal tubules
are ectatic, lined by attenuated epithelial
cells, and contain hypereosinophilic homogenous
material (protein casts) and cellular
debris. Multifocally the interstitium is
infiltrated by many lymphocytes and plasma
cells. Occasionally the interstitium is expanded
by lymphoid aggregates (lymphoid
hyperplasia and dysplasia). Some
glomerular tufts are senescent and shrunken
with ectatic Bowmans spaces.
Morphologic Diagnosis:
1.
Kidney, medium and small arteries:
Arteritis, proliferative and necrotizing,
multifocal.
2. Kidney, lymphoplasmacytic interstitial
nephritis, multifocal, mild.
3. Kidney, interstitial lymphoid hyperplasia
and dysplasia.
Lab Results:
N/A
Condition:
Polyarteritis nodosa-like syndrome/SIV
Contributor Comment:
Arteriopathy is
also noted in the small to medium arteries of
the mesentery, testis, liver, gall bladder,
pancreas, urinary bladder, and bone marrow.
The systemic vascular lesions in this
monkey resemble those found in polyarteritis
nodosa (PAN)-like syndrome in
HIV patients. PAN-like syndrome has been
described in HIV patients in the literature.
6,7
While target organs are usually muscles,
nerves, skin and gastrointestinal tract, renal
polyarteritis nodosa in HIV patients has also
been reported.2 PAN-like syndrome occurs
in fewer than 1% of HIV patients. The
underlying mechanism is thought to involve
cell or immune-complex-mediated inflammation,
like classic PAN in other
species. Although the histopathological
changes are similar between two entities,
there are several important differences
between PAN in HIV patients and so-called
classic or idiopathic PAN. First, the waxing
and waning clinical course of classic PAN is
not seen in patients with HIV infection.
Second, classic PAN can be associated with
hepatitis B virus infections, but in HIV
patients, the serology for HBV is always
negative. Third, the affected arteries in HIVassociated
PAN tend to be smaller than that
seen in classic PAN.
4
PAN-like syndrome has been reported in
two SIV-infected rhesus macaques.
11
Vasculopathy is prominent in kidney,
intestine, pancreas, liver, heart, lymph
nodes, spleen, and testis. Histologically, disseminated
arteriopathy is characterized by
intimal thickening and fibrosis with varying
degrees of vasculitis. Intranuclear inclusion
bodies were CMV positive by immunohistochemistry
in multiple organs in these
two monkeys. Intranuclear inclusion bodies
were not observed in the current case but
immunohistochemistry for CMV or other
viral agents was not performed in the current
case. Pulmonary arteriopathy is the
most common vasculopathy in
macaques infected with SIV.
Nineteen of 85 animals infected
with SIV developed pulmonary
arteriopathy characterized by
intimal thickening, luminal
occlusion, and internal elastic
laminae fragmentation and
interruption.
3
Pulmonary artery
hyperplasia and/or plexiform
arteriopathy were present in eight
of 13 (62%) SHIV-infected
macaques.
5 However, the
pulmonary arteries were histopathologically
normal in the
current case. This observation is
consistent with the two published
cases, in which, arteriopathy was
mild or absent in the lungs.
11
These observations suggested a
different pathogenesis
between pulmonary
arteriopathy and PANlike
syndrome in SIV
infected monkeys.
Based on extensive experience on this
model, it is unlikely that ethanol administration
was associated with PAN-like
syndrome in this monkey. There is no
documentation of alcohol and arteriopathy
in the literature. Although this animal was
inoculated with Streptococcus pnenumonae,
grossly and microscopically there was no
current evidence of Streptococcus infection.
Renal interstitial lymphoid hyperplasia and
dysplasia are not uncommon findings in
SIV-infected monkeys.
JPC Diagnosis:
1. Kidney, small arteries
and arterioles: Arteriopathy, proliferative
and necrotizing, multifocal, mild to marked,
with adventitial inflammation, rhesus
macaque (
Macaca mulatta).
2. Kidney: Interstitial nephritis, lymphoplasmacytic,
multifocal, mild.
Conference Comment:
Although the
specific etiology and pathogenesis of this
lesion are unclear, the contributor provides
an excellent example of a polyarteritis
nodosa (PAN)-like syndrome in a nonhuman
primate. PAN-like syndromes are
thought to be a type III hypersensitivity
reaction secondary to antigen:antibody
complex deposition in medium to small
caliber arteries.
1 Immune complex
deposition results in complement activation
leading to segmental, circumferential, and
proliferative arteritis. This syndrome has
been well described in the aged SpragueDawley
rat and beagles.
9,10 In rats, lesions
most often occur in the muscular mediumsized
arteries of the mesentery, pancreas,
testis, hepatic, coronary, uterine, cerebral,
adrenal, and renal arteries.
10 This condition
in beagles is associated with beagle pain
syndrome. In these cases, the coronary and
meningeal arteries are most affected, and
clinically dogs are febrile, lose weight, and
have cervical pain.
9 Typically in domestic
species, PAN-like syndrome spares the
pulmonary circulation, large arteries and
glomeruli.
11 The association of SIV as part
of the pathogenesis of the arteriole lesions,
in this case, remains unclear.
The JPC strives to avoid using the suffix -
opathy in a morphologic diagnosis due to
its non-specific nature; however, in rare
instances, this terminology may be
appropriate, especially in cases where the
primary process underlying the lesion is
difficult to ascertain. While the SIV-positive
status of this particular animal suggests a
causal relationship, PAN has also been seen
as a spontaneous finding in macaques, as
well as a toxic lesion association with
administration of cyclosporine and
tacrolimus (WSC 2003-2004, Conference
20, Case 1). The term arteriopathy can be
modified by other descriptors such as
proliferative and necrotizing to further
define the underlying process. Much of the
literature on this disease uses the term
arteriopathy to describe this finding in
arteries and arterioles in SIV-infected rhesus
macaques.
During a discussion of the vessel wall
changes in this case, some conference
participants preferred the term hyaline
change to describe the circumferential
homogenous, eosinophilic, proteinaceous
material deposited within the external elastic
membrane of arterioles rather than the wellensconced
term fibrinoid necrosis. Fibrinoid
necrosis has been classically used by both
human and veterinary pathologists to
describe the brightly eosinophilic changes in
the injured vessel associated with immune
complex, plasma protein, and complement protein deposition within vessels.
1 Fibrinoid
necrosis implies a pathogenesis that may or
may not be present. The brightly
eosinophilic homogenous protein accumulation
obscures the structural detail of the
blood vessel, thus making it difficult or
impossible to determine if there is fibrin or
necrosis present within the arteriole wall.
Hyalinosis describes the accumulation of
leaked eosinophilic proteinaceous material
secondary to endothelial damage and increased
vascular permeability without
making assumptions about the pathogenesis.
Finally, several participants found multinucleated
giant cells within the tubular
epithelium and lumina of collecting ducts
within their sections. Multinucleated giant
cells have been described as a common
incidental finding in macaques
8
; however, a
number of participants ascribed them as a
potential corroborating sign of lentivirus
infection in this macaque.
References:
1. Alpers C, Chang A. The kidney. In:
Kumar V, Abbas AK, Fausto N,
Aster JC, eds.
Robbins and Cotran
Pathologic Basis of Disease. 9th ed.
Philadelphia, PA:Saunders Elsevier;
2015:903.
2. Angulo JC, Lopez JI, Garcia ME,
Peiro J, Flores N: HIV infection
presenting as renal polyarteritis
nodosa.
Int Urol Nephrol. 1994;
26(6):637-641.
3. Chalifoux LV, Simon MA, Pauley
DR, MacKey JJ, Wyand MS, Ringler
DJ: Arteriopathy in macaques
infected with simian immunodeficiency
virus.
Lab Invest.
1992:67(3):338-349.
4. Chetty R: Vasculitides associated
with HIV infection.
J Clin Pathol.
2001; 54(4):275-278.
5. George MP, Brower A, Kling H,
Shipley T, Kristoff J, Reinhart TA, et
al.: Pulmonary vascular lesions are
common in SIV- and SHIV-envinfected
macaques.
AIDS Res Hum
Retroviruses. 2011; 27(2):103-111.
6. Gisselbrecht M, Cohen P, Lortholary
O, Jarrousse B, Gayraud M,
Lecompte I, et al.: Human immunodeficiency
virus-related vasculitis.
Clinical presentation of and
therapeutic approach to eight cases.
Ann Med Interne. 1998; 149(7):398-
405.
7. Libman BS, Quismorio FP, Jr.,
Stimmler MM: Polyarteritis nodosalike
vasculitis in human immunodeficiency virus infection.
J
Rheumatol. 1995; 22(2):351-355.
8. Lowentine, L.J. A primer of primate
pathology lesions and nonlesions.
Tox Pathol 2003; 31:91-102.
9. Miller L, Van Vleet J, Gal A.
Cardiovascular system and
lymphatic vessels. In: McGavin MD,
Zachary JF, eds.
Pathologic Basis of
Veterinary Disease. 5th ed. St.
Louis, MO:Mosby Elsevier;
2012:587.
10. Percy DH, Barthold SW. Rat. In:
Pathology of Laboratory Rodents
and Rabbits, 4th ed., Ames, IA:
Blackwell Publishing; 2016:156.
11. Robinson W, Robinson N. Cardiovascular
system. In: Maxie MG, ed.
Jubb, Kennedy, and Palmers
Pathology of Domestic Animals. Vol
3. 6th ed. Philadelphia, PA:Elsevier;
2016:71.
12. Yanai T, Lackner AA, Sakai H,
Masegi T, Simon MA: Systemic
arteriopathy in SIV-infected rhesus
macaques (Macaca mulatta).
J Med
Primatol. 2006; 35(2):106-112.