Wednesday Slide Conference, Conference 6, Case 2
Signalment:
12-week-old male ABCA4 -/- mouse.
History:
A 12-week-old male ABCA4 -/- mouse was presented for routine necropsy; no associated clinical signs were reported.
All procedures performed on animals were in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee or through an ethical review process.
Gross Pathology:
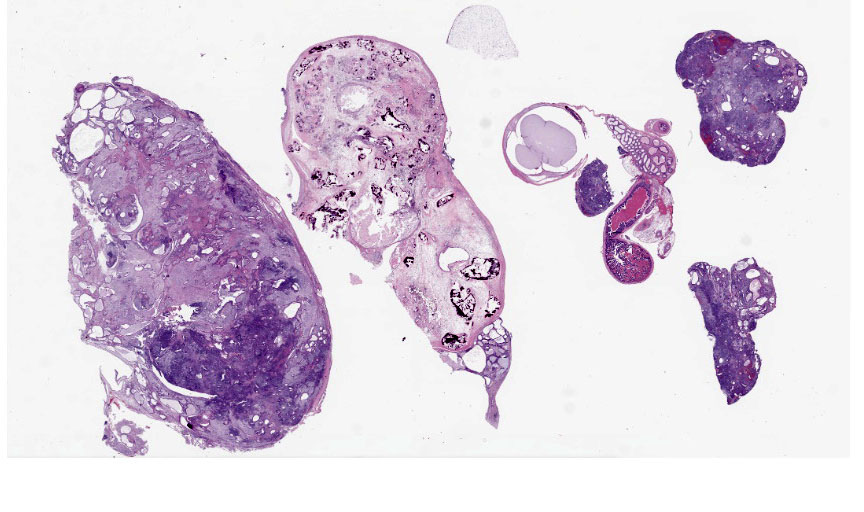
Expanding the abdominal cavity and displacing abdominal organs were multiple variably sized irregular masses. The masses were pale tan and smooth.
Microscopic Description:
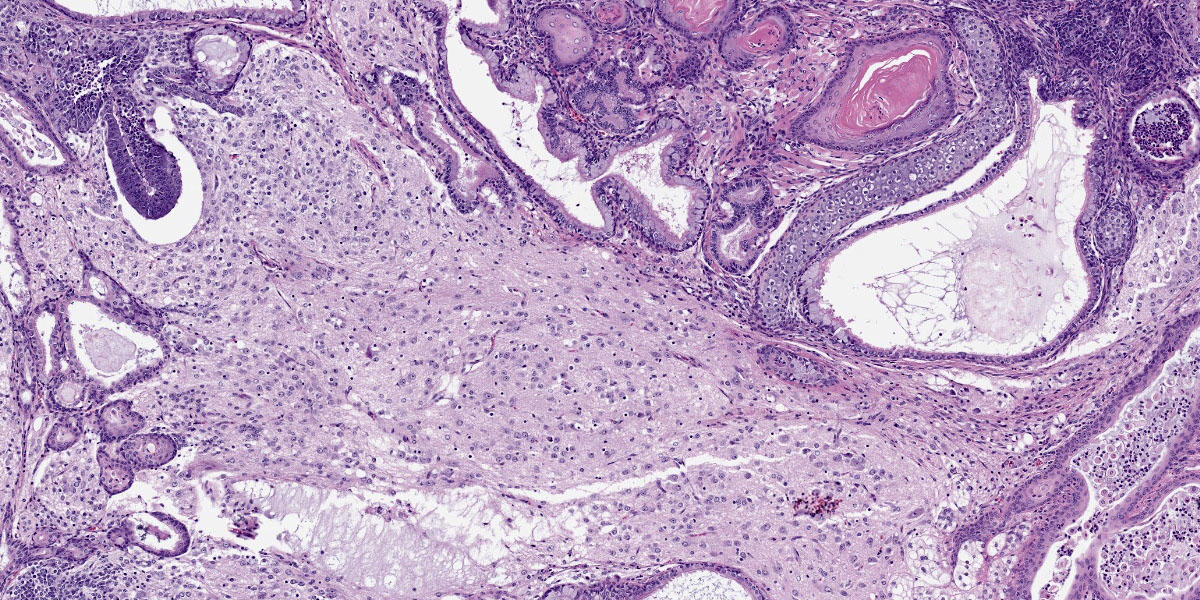
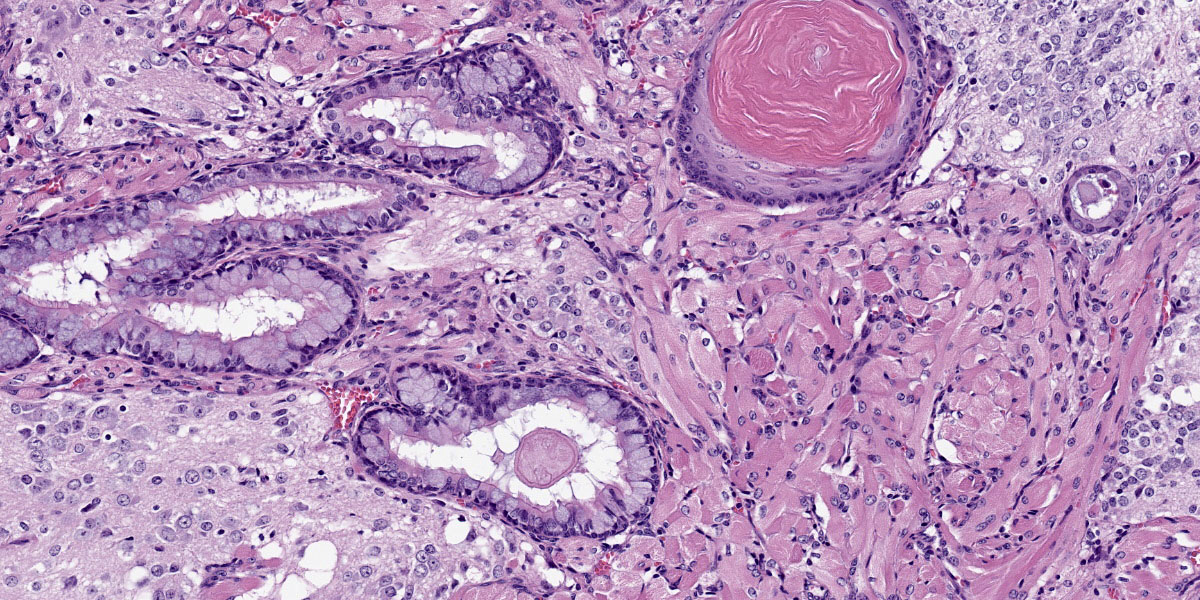
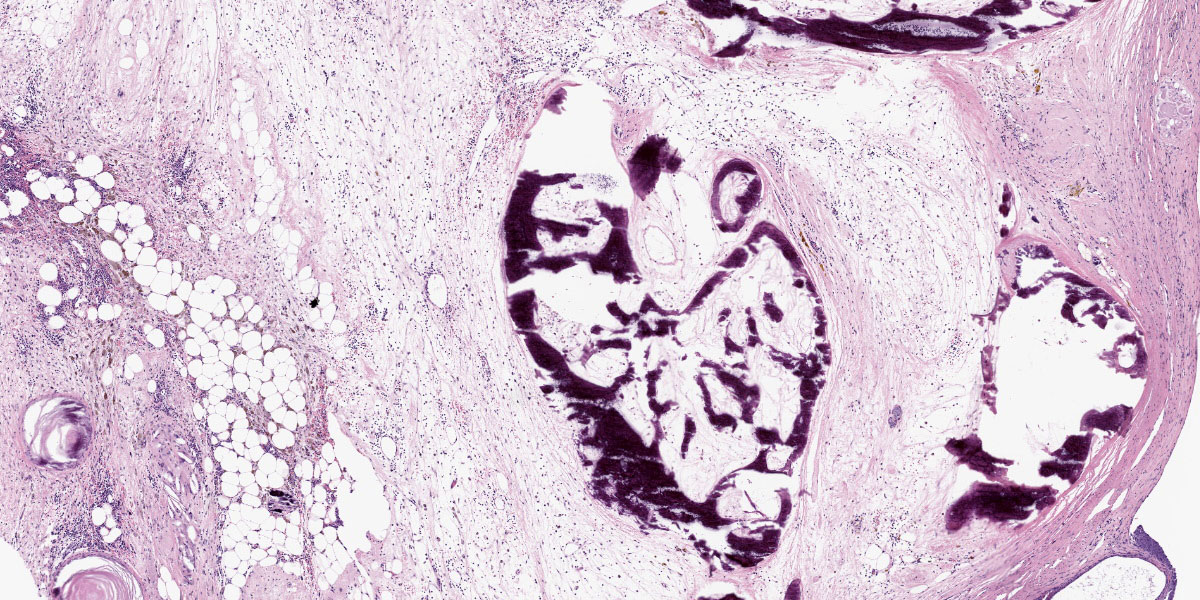
Mass: Loosely associated with the seminal vesicle is a well-demarcated, unencapsulated, multinodular neoplastic mass. The densely cellular to cystic neoplasm is composed of variably differentiated neoplastic cells from three, depending on the section, germ cell layers and multiple tissue types. Ectodermal tissue includes neural tissue, including neurons and glial cells, surrounded by neuropil, and multifocal nests and cysts of squamous epithelium with variable keratinization. In addition, fusiform cells arranged in rosettes, suggestive of neuroendocrine origin, are scattered throughout the mass. Endodermal tissue includes multifocal variably sized tubules lined by cuboidal to columnar epithelial cells. Columnar epithelial cells are often pseudostratified and have apical cilia (respiratory epithelium); goblet cells are interspersed. Mesodermal tissue includes multifocal bundles of smooth muscle, multifocal foci of osteocytes embedded in eosinophilic osteoid and depending on the section, foci of calcified bone, adipose tissue, and rare aggregates of chondrocytes embedded in chondroid matrix (consistent with cartilage). On average, there are less than 1 mitotic figures per high power field.
Contributor’s Morphologic Diagnosis:
Abdominal mass, teratoma.
Contributor’s Comment:
Although uncommon in domestic animals, teratomas have been reported in a wide variety of veterinary species. In domestic animals teratomas of the testis have been occasionally reported in foals7, while in laboratory animals, teratomas have been reported in mice, rats, ferrets, and non-human primates.3,4,8,13 In certain inbred mouse strains, notably 129 mice and its substrains, teratomas have been recognized in the veterinary literature as spontaneous neoplasms since 1954.4,12 In the 129-mouse strain, a ter mutation is responsible for the increase in teratoma incidence.4
Thought to arise from multipotent germ cells, teratomas have been reported in multiple tissues including brain, adrenal glands, gonads, uterus.2,4,10,13 In the nervous system, suprasellar germ cell tumors, like teratomas are most commonly identified on midline.2 The presence of tissue derived from more than one germinal layer, ectoderm, endoderm, and/or mesoderm, is a requirement for a teratoma diagnosis.1,7,11 The presence of two or more germinal layers is a result of the initial somatic differentiation of germ cells, giving rise to a variety of tissues that can be present in these tumors.11 In females, ovarian teratomas are considered parthenogenic tumors, as they are thought to arise from a single germ cell, which has undergone an initial round of meiotic division, but not a second.11
Macroscopically, teratomas can be solid and/or cystic, and occasionally contain obvious hair, bone, or teeth.7,11 Histologically, numerous tissue types have been reported to be components of teratomas, and these various tissue elements can be either mature or immature.6 In this case, nervous tissues, squamous epithelium, respiratory epithelium, adipose tissue, smooth muscle, bone, and cartilage were all present. Nervous tissue, adipose tissue, and respiratory epithelium are commonly found in teratomas.7
Benign teratomas are characterized by well differentiated tissue, with no significant hemorrhage or necrosis.5 Benign teratomas do not invade surrounding tissue and do not metastasize, while malignant teratomas have evidence of local invasion and/or metastasize.4,5 At gross necropsy of this ABCA4-/- mouse, multiple masses were scattered throughout the abdomen, consistent with carcinomatosis and malignant behavior. Although standard histologic evaluation of teratomas is likely sufficient for diagnosis, immunohistochemistry can be employed to highlight the different tissue types.
Contributing Institution:
Pfizer Research and Development
455 Eastern Point Rd.,
Groton, CT 06340
www.pfizer.com
JPC Diagnosis:
Caudal abdominal mass: Teratoma
JPC Comment:
Teratomas are a regular submission to the WSC (Conference 1, Case 4, 2023-2024 and Conference 16, Case 2, 2020-2021). Case 2 is a classic teratoma with a wide array of recognizable tissues from multiple germ cell layers (figures 2-3 through 2-5. Although not needed for this case, histo- and immunohistochemical stains can assist in the recognition of certain tissues. In this case, cartilage is nicely outlined by an Alcian blue pH 1.0 while PAS highlights mucus within goblet cells and basement membranes adjacent to epithelial tissues. Conference participants noted ancillary features such as an inflammatory exudate (sans bacteria) within the cysts lined by respiratory epithelium (however, we refrained from diagnosis any type of pneumonia in this case). There was brief discussion of the potential behavior of this teratoma based on its morphology; participants agreed that the morphology appeared more “benign” than the history provided by the contributor. Other recent WSC submissions have featured more clear examples of malignant behavior with invasion of adjacent issues.
Case reports of teratomas have recently been published in cats and in birds.9,14 Extragonadal teratomas are unusual in domestic animals; two recent feline cases dealt with oropharyngeal teratomas.14 Similar to human congenital oropharyngeal teratomas, affected cats were young and had masses near the sphenoid bone consistent with an origin from Rathke’s pouch. In contrast, a coelomic teratoma was described in a 26 year-old eclectus parrot. 9 Despite the mass being well-differentiated, the large size was sufficient to induce increased respiratory effort, lethargy, regurgitation, and adhesions to multiple organs. Although the mass was successfully resected, the bird decompensated and died after surgery.9 This is an effective reminder that benign neoplasms can still have significant effects on morbidity and mortality.
References:
- Agnew DW, MacLachlan NJ. Tumors of the Genital Systems. In: Meuten DJ, ed. Tumors in Domestic Animals, 5th ed. 2017; Ames, IA, John Wiley & Sons, Inc., p. 689-722.
- Cantile C, Youssef S. Nervous System, In: Maxie MG, ed. Jubb Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed. 2016; St. Louis MO, Elsevier Press, vol 1, p. 404.
- Cline JM, Wood CE, Vidal JD, et al. Selected Background Findings in Interpretation of Common Lesions in the Female Reproductive System in Macaques. J Toxicol Pathol 2008; 142S-163S.
- Creasy D, Bube A, de Rijk E, et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Male Reproductive System, J Toxicol Pathol 2012; 40: 40S-121S.
- Dixon D, Alison R, Bac U, et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Female Reproductive System. J Toxicol Pathol 2014; 27 (3 & 4 Supplemental):1S-107S.
- Epstein JI, Lotan TL. The Lower Urinary Tract and Male Genital System. In: Kumar V et al. ed. Robbins and Cotran Pathologic Basis of Disease. 9th ed. 2015; Philadelphia, PA, Elsevier Saunders. p. 959-990.
- Foster RA. Male Genital System, In: Maxie MG, ed. Jubb Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed. 2016; St. Louis MO, Elsevier Press, vol 3, p. 465-510.
- Kirejczyk S, Pinelli C, Gonzalez O, et al. Urogenital Lesions in Nonhuman Primates at Two National Primate Research Centers. Vet Pathol. 2021 January; 58(1): 147-160.
- Mayer CC, Richard JN, Lin CM, Conrado FO, Hahn S, Graham JE, Bercier M. Intracoelomic Teratoma in an Eclectus Parrot (Eclectus roratus). J Avian Med Surg. 2021 Jul;35(2):217-226.
- Ogata K, Masahiko K, Miyata K et al. Well-Differentiated Teratoma in a Mouse Uterus. J Toxicol Pathol 2011; 39: 901-904.
- Schlafer DH and Foster RA. Female Genital System, In: Maxie MG, ed. Jubb Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed. 2016; St. Louis MO, Elsevier Press, vol 3, p. 359-464.
- Stevens LC and Little CC. Spontaneous Testicular Teratomas in and Inbred Strain of Mice. Proc Natl Acad Sci USA. 1954 Nov; 40(11): 1080-1087.
- Williams BH, Yantis LD, Craig SL, et al. Adrenal Teratoma in Four Domestic Ferrets (Mustela purorius furo). Vet Pathol 2001; 38: 328-331.
- Yuzbasioglu-Ozturk G, Gulcubuk A, Ozturk-Gurgen H, Demirutku A, Akcasiz ZN, Ozkul S. An unusual case of oropharyngeal mature teratoma in a kitten. Iran J Vet Res. 2023;24(4):365-368.