Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 6
October 4th, 2017
CASE I: 3120125021 (JPC 4017935).
Signalment: 1-year-old, Crested Guinea fowl, Guttera pucherani, avian.
History: A peri-orbital mass surrounded the left eye. Growth of the mass initially started rostrally from the eye and slowly extended to the nose and the upper eyelids. Initial treatment with enrofloxacin gave transient improvement. A second treatment with the same antibiotic did not have effect. Bacterial culture yielded Staphylococci susceptible to doxycyclin. Treatment with doxycyclin was started but did not give improvement. A surgical biopsy was then taken and submitted for histopathology.
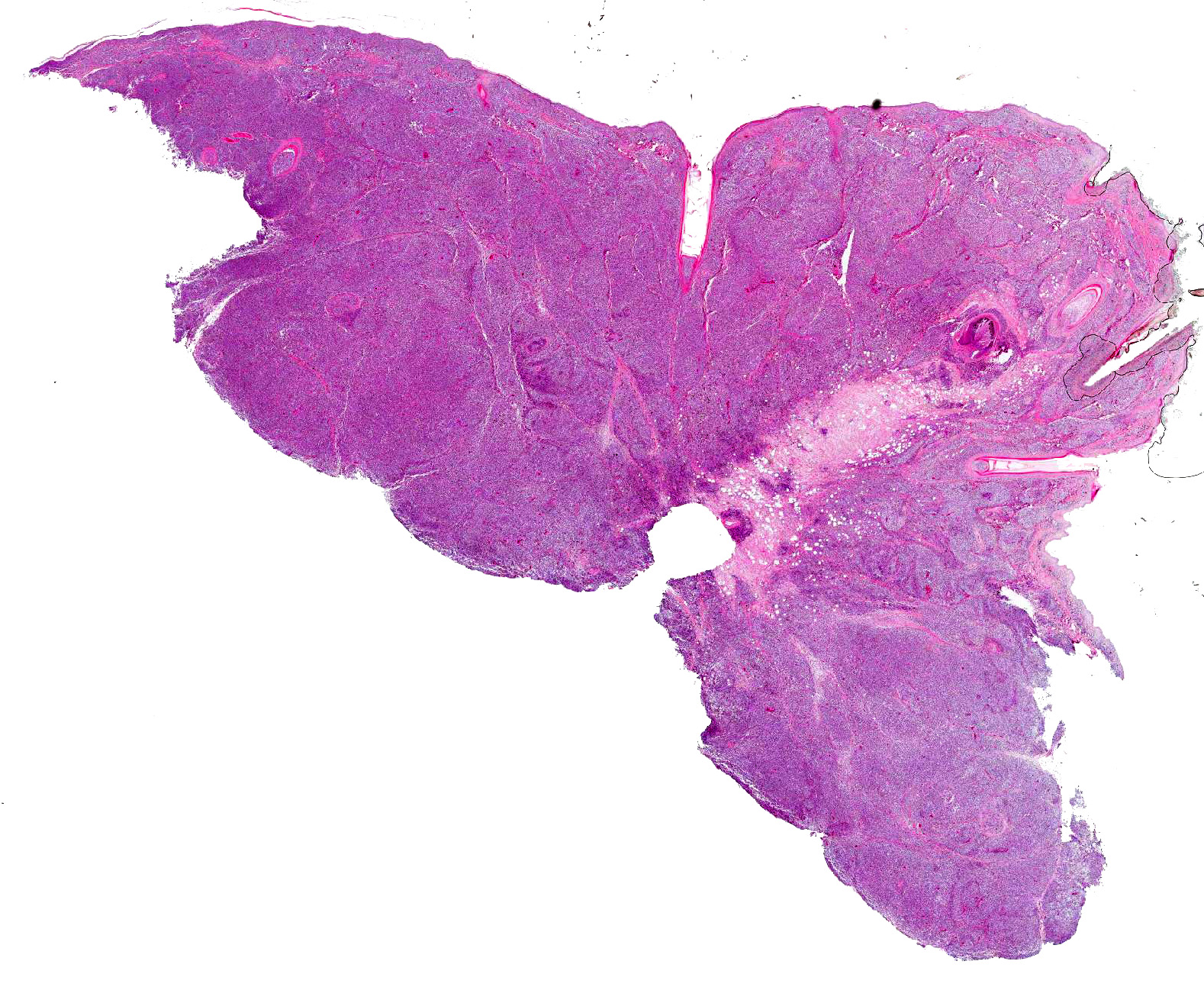
Gross Pathology: Bilaterally there was a mass of approximately 1.5cm in diameter, involving the upper eyelids and extending into the subcutis of the nose. On cut surface the mass was solid, firm and marbled pale tan to black with multifocally softer tissue with cystic spaces containing viscous mucoid material.
Laboratory results: Bacterial culture in the past: Staphylococci, not further specified.
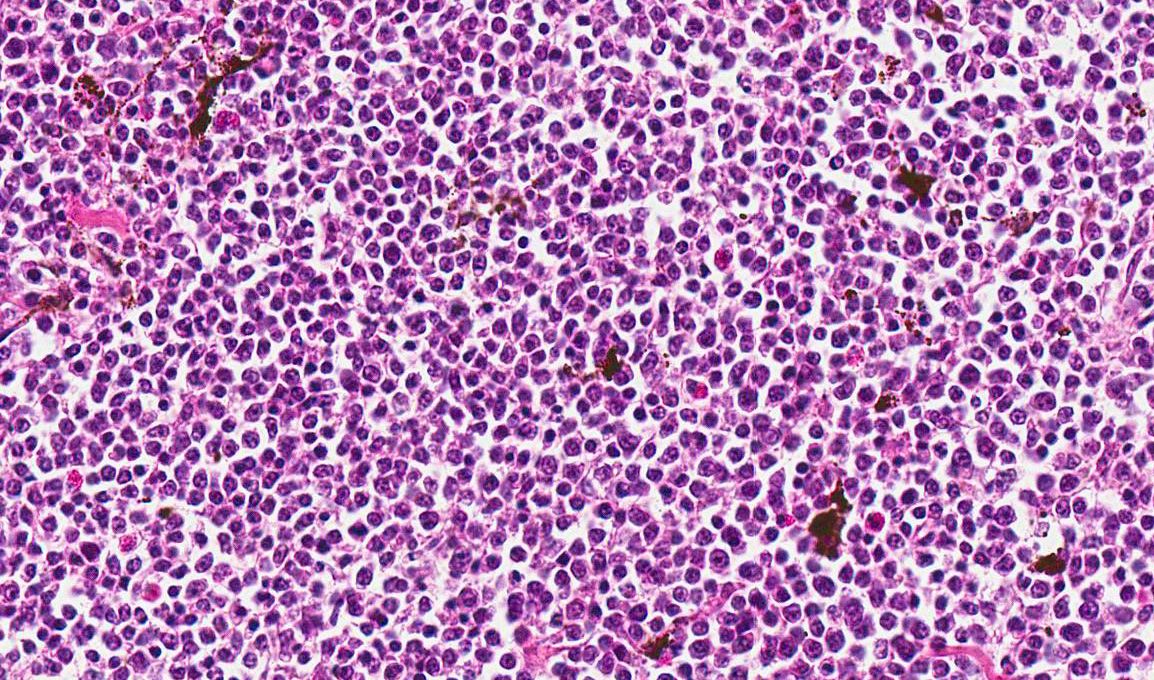
Microscopic Description: Skin: In the dermis and subcutis, elevating the overlying epidermis, is a densely cellular, poorly demarcated, non-encapsulated, infiltrative growing, monomorphic round cell neoplasm. Neoplastic cells are organized in poorly defined lobules of large solid sheets with scant intermingled fibrovascular connective tissue. Neoplastic cells are round to oval with a small amount of pale amphophilic cytoplasm and indistinct cell borders. Nuclei are round and contain coarsely clumped chromatin and one or two moderately distinct nucleoli. Mitotic figures are 18 per 5 HPF and there is mild anisocytosis and anisokaryosis. Small numbers of neoplastic cells multifocally infiltrate the epidermis and feather follicle epithelium and theres multifocal infiltration and replacement of pre-existing muscle tissue and lacrimal glands (not present in all slides). Multifocally there are areas of necrosis characterized by pyknosis, karyorrhexis, karyolysis, loss of cellular detail and amorphous, eosinophilic debris with associated infiltration of moderate numbers of heterophils. Multifocally dispersed throughout the neoplasm there are small numbers of heterophils and macrophages with an occasional multinucleated giant cell. The overlying epidermis is multifocally ulcerated and covered with serocellular crusts (not present in all slides).
Contributors Morphologic Diagnosis:
Skin: Lymphoma.
Contributors Comment: Guinea fowl are terrestrial, ground-living birds that belong to the order Galliformes, family Numidae. Some ornithologists consider them to be part of the superfamily Phasanidae (including Partridges and Pheasants).
There are limited reports available on lymphoid neoplasms and neoplasms in general in guinea fowl. Pancreatic adenocarcinomas and seminomas have been reported as rare spontaneous neoplasms in guinea fowl.3,1 Viral- induced pancreatic tumors and duodenal adenomas have been reported in guinea fowl in experimental settings.9,2 In general, the most common naturally-occurring neoplasms in birds are lymphomas, fibromas and fibrosarcomas, and lipomas6; howeve,r these information are based upon a population including species of the Galliformes order but not including domestic fowl.
Lymphomatous tumors are also relatively common in domestic fowl and turkeys. Common sites of occurrence are liver, spleen, thymus and kidneys. Cutaneous and oropharyngeal lymphomas have been reported in pheasants. The neoplasms were present in the skin around the eyes, around the external ear openings and involving the hard palate.4 Histologically, these tumors were composed of a pleomorphic mixture of lymphoblasts and lymphocytes. 4
A similar case of a guinea fowl presenting with a periorbital lymphoma has been reported and was associated with visceral lymphomas in the spleen, lungs, kidneys, liver and ovary. 8 In our case, however, we did not perform a full necropsy and information regarding the internal organs was not available.
Most information on ocular and periorbital tumors in animals is from dogs and cats. In these animals, ocular tumors are relatively rare. Ocular tumors can arise from the eyelids and adnexa, the optic nerve and structures within the globe, and metastases are generally infrequent. The most frequent neoplasms of the eyelid and conjunctiva in dogs are squamous cell carcinoma and Meibomian adenoma. Less frequently encountered are melanocytic neoplasms, papillomas, vascular tumors, mast cell tumors and lymphomas.
In dogs, cats and cattle, multicentric lymphoma regularly involves the eye. In dogs and cats, there is predominant involvement of the uvea, whereas in cattle retrobulbar (orbital) neoplasms are more common. Only occasionally are lymphomas encountered in the tissues of the eyelids in these animals. Solitary lymphomas in the conjunctiva and third eyelid have been reported in cats and horses.
In birds, squamous papillomas, squamous cell carcinomas, malignant melanomas, basal cell tumors and adeno(carcino)mas of the lacrimal gland have been reported in the skin and subcutis of the eyelids.7 Lymphoma is reported as a neoplasm arising in the orbit as are infiltrative carcinomas, chondromas and teratomas.7 An infectious etiology has not been identified in the lymphomas reported in pet birds or in the report of a periorbital lymphoma in a Guinea fowl.7,8
Lymphocytic
neoplasms in poultry are often categorized as infectious or non-infectious.
Spontaneously occurring neoplasms often involve older birds whereas viral
induced neoplasms develop in relatively young birds.5 The most
important viral-induced neoplastic diseases in poultry are:
- Mareks disease, caused by Gallid herpesvirus type 2
- Avian Leukosis caused by avian leukosis virus (retrovirus)
- Reticuloendotheliosis, caused by
reticuloendotheliosis virus (retrovirus).
Of these entities, Mareks disease can involve the skin.5 Mareks disease is a disease that mainly affects chickens and only occasionally affects pheasants, quail, game fowl and turkeys. To the best knowledge of the author, Mareks disease has not been reported in guinea fowl.
JPC Diagnosis: Feathered skin: Lymphoma, crested guinea fowl (Guttera pucherani), avian.
Conference Comment: There are three main viral neoplastic diseases in chickens which result in lymphoid tumors: Mareks disease (MD), avian leukosis, and reticuloendotheliosis.5
Mareks disease is caused by an alpha herpesvirus (Gallid herpesvirus-2) and typically affects young chickens, and rarely quail, turkeys, pheasants, and jungle fowl. The herpesvirus that causes MD is classified into three serotypes. Serotype 1 is ubiquitous in chickens and varies in pathogenicity from very virulent (vv+) which is oncogenic to avirulent (mild). Serotype 2 is also common in chickens but is non-oncogenic and serotype 3 is common in turkeys and also non-oncogenic. The virus is spread through inhalation of virus-containing feather follicle dander of infected birds which can spread across long distances. Carrier birds can be silently infected and periodically shed the virus throughout their lives. There are four different types of lesions seen in MD: (1) peripheral nerve enlargement, (2) discoloration of the iris; (3) swelling of feather follicles with skin reddening (leukosis); (4) and visceral tumors often involving heart, spleen, liver, gonads, kidneys, and proventriculus. Of the four, visceral tumors are most common and can result in depressed, cachexic birds prior to death with vague clinical signs. Microscopically, lymphomas caused by MD contain pleomorphic T-lymphocytes that carry a MD tumor-associated surface antigen (MATSA).5 In pet birds, epitheliotropic lymphoma that appears similar to MD occurs but the etiology has not been identified.11
Avian leukosis (ALV) affects mature chickens and is caused by alpha retroviruses known as avian leukosis viruses which have been further classified into 10 subgroups (A through J). Retroviral strains are classified by the pathogenicity of their lesions and subgroup. Lymphoid leukosis (LL) is the most common, caused by ALV subgroup A, and characterized by gradual onset, low mortality, and neoplasia of the Bursa of Fabricius with metastasis to other visceral organs. Recently a new strain of ALV was discovered, J, which causes myeloid leukosis (myelocytomatosis) and most likely results from recombination of endogenous and exogenous retroviruses. In contrast to MD, egg transmission is the predominate mechanism of spread in ALV. These chicks are immune tolerant and do not develop antibody but have an increased risk of death from LL. If female, these chickens will lay fewer eggs and shed the virus to their own progeny further disseminating the infection. Clinical signs of ALV are non-specific and many birds simply appear emaciated and lethargic. Tumors may be detected within the bursa of Fabricius by insertion of the clinicians finger into the cloaca. Additionally, birds with skeletal myelomatosis (subgroup J) may develop osteopetrosis of the shanks resulting in what are colloquially known as boot shanks. Avian retroviruses cause osteopetrosis by infecting osteoblasts and making them constitutively active, while osteoclasts remain unaffected.1,2 Microscopically, neoplastic cells are uniformly lymphoblastic and positive for immunoglobulin M and B-cell markers since they originate in the Bursa of Fabricius.5
Reticuloendotheliosis (RE) encompasses a variety of conditions caused by retroviruses. Only two conditions caused by this non-defective RE virus, runting syndrome and chronic lymphoma are of economic importance particularly in the southern U.S. Runting disease is induced by vaccination with RE virus-contaminated biologics of chicks less than 1 week old and characterized by stunted growth, feather abnormalities, and severe atrophy of the thymus and Bursa of Fabricius leading to immunosuppression and concurrent infections. Chronic lymphoma syndrome occurs very rarely in turkeys, ducks, quail, pheasants, geese, peafowl, prairie chickens and chickens and results in few clinical signs other than mild depression prior to death. In experimentally infected animals lesions resemble LL or MD.5
Diagnosis of lymphoid tumors in birds can be difficult and necessitates a complete history, thorough necropsy, immunofluorescent tests for surface antigens, and molecular techniques like PCR.5
http://www.uu.nl/faculty/veterinarymedicine/EN/labs_services/vpdc
References:
1. Foster RG, Lian JB, Stein G, Robinson HL. Replication of an osteopetrosis-inducing avian leukosis virus in fibroblasts, osteoblasts, and osteopetrotic bone. Virology. 1994;205(1):179-187.
2. Foster RG, Robinson HL. Establishment of interference in osteoblasts by an osteopetrosis-inducing avian leukosis virus. Virology. 1994;205(1):376-378.
3. Golbar H. M, Izawa T, Kuwamura M et al. Malignant seminoma with multiple visceral metastases in a Guinea Fowl (Numida meleagris) kept in a zoo. Avian Diseases Digest. 2009;4(1):143-145.
4. Kirev T, Toshkov IA, Mladenov ZM. Virus induced duodenal adenomas in guinea fowl. J. Natl. Cancer. Inst. 1987;79 (5):1117-1121.
5. Ojkic D, Brash ML, Jackwood MW, Shivaprasad HL. Viral diseases. In: Boulianne M, ed. Avian Disease Manual. 7th ed. Jacksonville, FL: American Association of Avian Pathologists, Inc.; 2013:30-38.
6. Okoye JOA, Ilochi CC. Pancreatic adenocarcinoma in guinea fowl. Avian Pathology 1993;(22):401-406.
7. Pattison M, McMullin PF, Bradbury JM, Alexander DJ. Poultry Diseases. 6th ed. Philadelphia, PA: Elsevier; 2008:569-570.
8. Payne LN, Venugopal K. Neoplastic diseases: Mareks disease, avian leukosis and reticuloendotheliosis. Rev. sci. tech. Off. Int. Epiz. 2000;19(2): 544-564.
9. Reece RL. Observations on naturally occurring neoplasms in birds in the state of Victoria, Australia. Avian Pathology 1992;(21):3-32.
10. Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. 1st ed. Ames, IA: Iowa State Press; 2003:206-207.
11. Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. 2nd ed. Ames, IA: Iowa State Press; 2015:258-259.
12. Schnellbacher RW, Wilson S. What is your diagnosis?. Journal of Avian medicine and Surgery 2010;24(3):241-244.
13. Toshkov I, Kirev T, Bannasch P. Virus induced pancreatic cancer in guinea fowl. An electron microscopical study. Int J Pancreatol. 1991;(1):51-64.