CASE III:
Signalment:
Five year old intact male ferret (Mustela putorius furo), mustelid.
History:
This is the second ferret from a rescue facility to present to the referring veterinarian in a severely debilitated state, emaciated, dehydrated and hypothermic. The ferret was euthanized.
Gross Pathology:
This male ferret was in emaciated body condition, with a poor, sparse hair coat and hair loss of the distal tail. Internally, there were still some subcutaneous adipose stores in the inguinal fatpad, around the kidneys and in the mesentery, however there was generalized muscle wasting. The heart was globose. The adrenal glands were small and symmetrical. Small intestinal content was scant, and there were soft dark brown feces in the colon.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
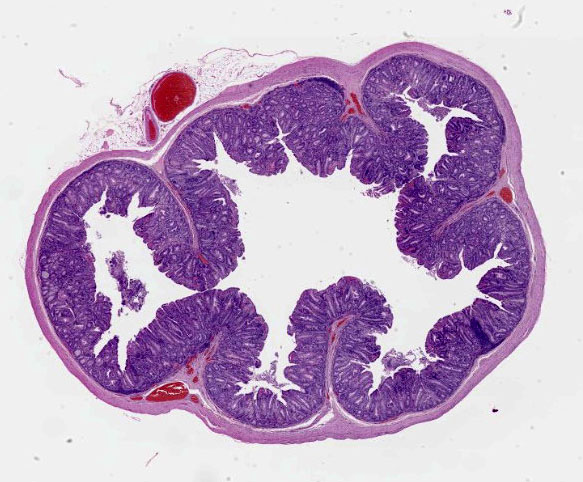
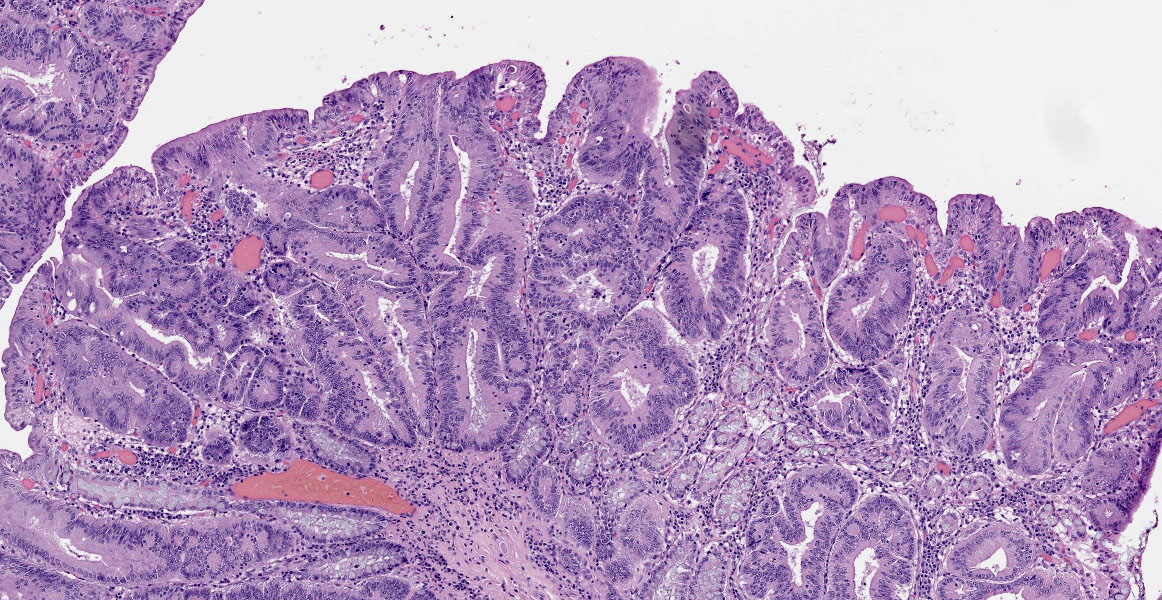
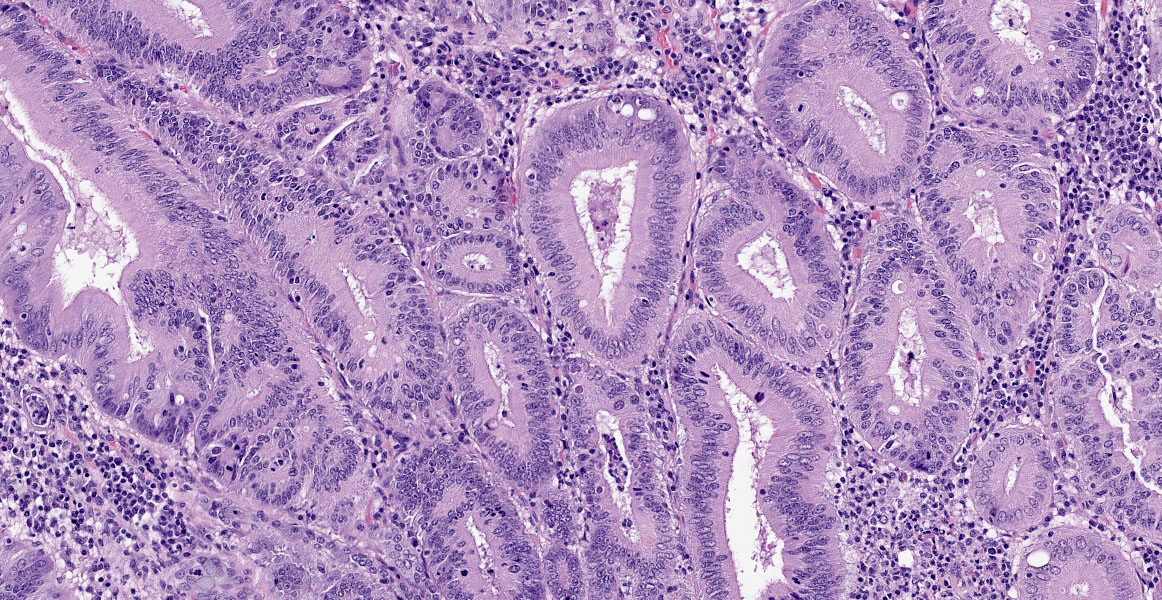
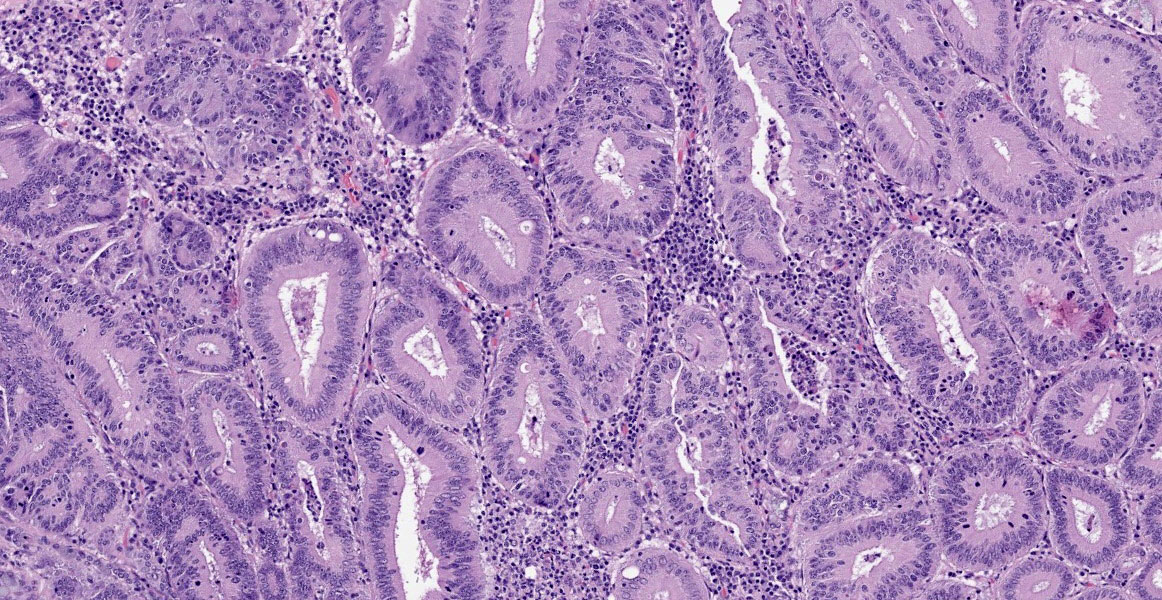
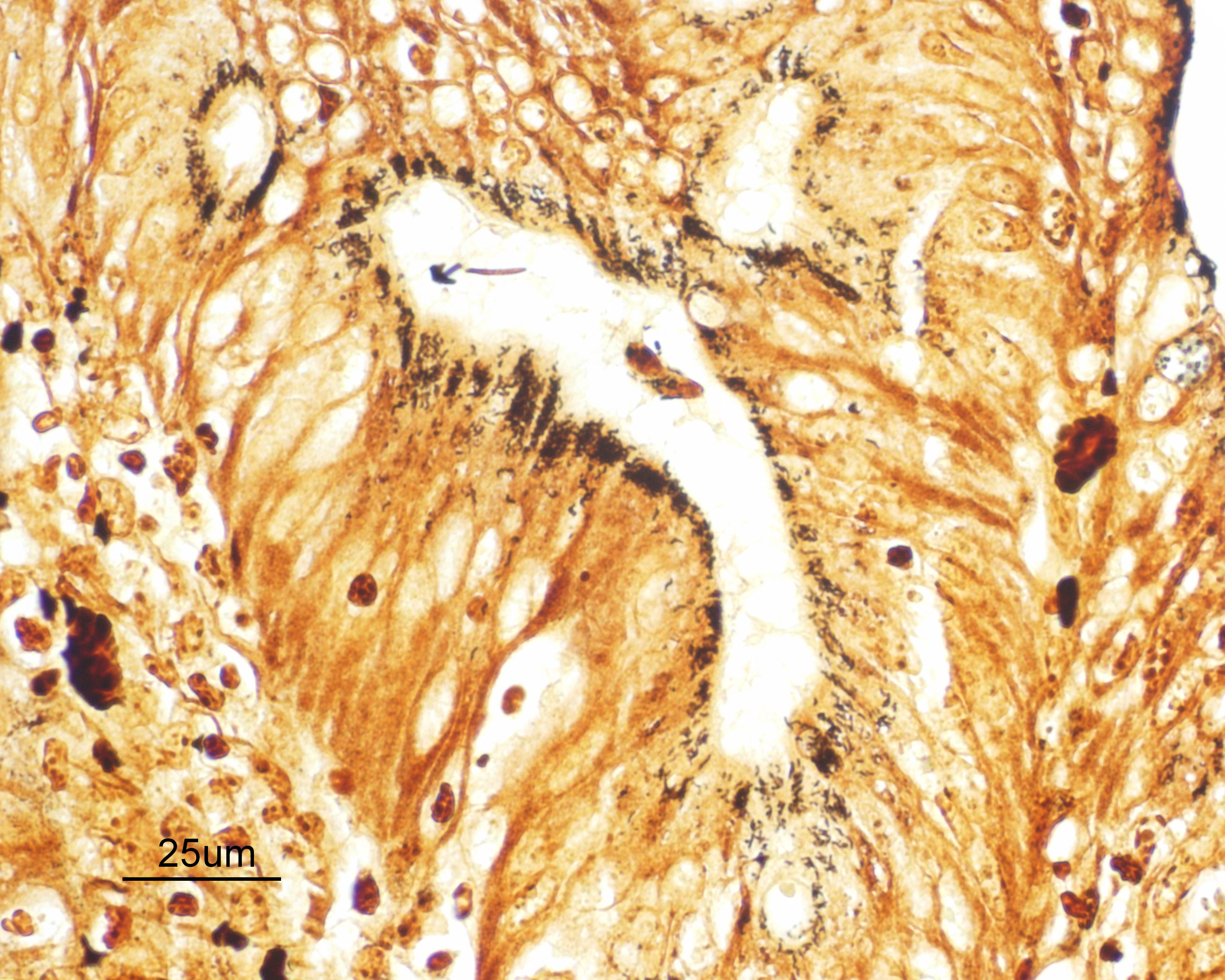
Small intestine: Diffusely throughout the section, there is villus blunting and fusion with loss of goblet cells and marked cryptal epithelial hyperplasia, characterized by elongated and tortuous crypts lined by tall columnar epithelial cells with abundant lightly eosinophilic cytoplasm and elongate vesicular nuclei. The crypt epithelium is disorganized and occasionally piles up to five cells thick at the base of the crypts. There is frequent single cell death within the mucosal epithelium, characterized by individualized and hypereosinophilic enterocytes with fragmented or condensed nuclei; mitotic figures are also frequent. Occasional crypt lumens are dilated, lined by attenuated low columnar to cuboidal epithelium, and contain necrotic cellular debris or mucinous fluid. The lamina propria and submucosa are variably expanded by clusters of lymphocytes, plasma cells and macrophages. The villus epithelium is predominantly intact, multifocally attenuated at the surface, and contains scattered apicomplexan organisms of various life stages, including meronts, macrogametocytes, microgametocytes and occasional oocysts visible within the luminal debris.
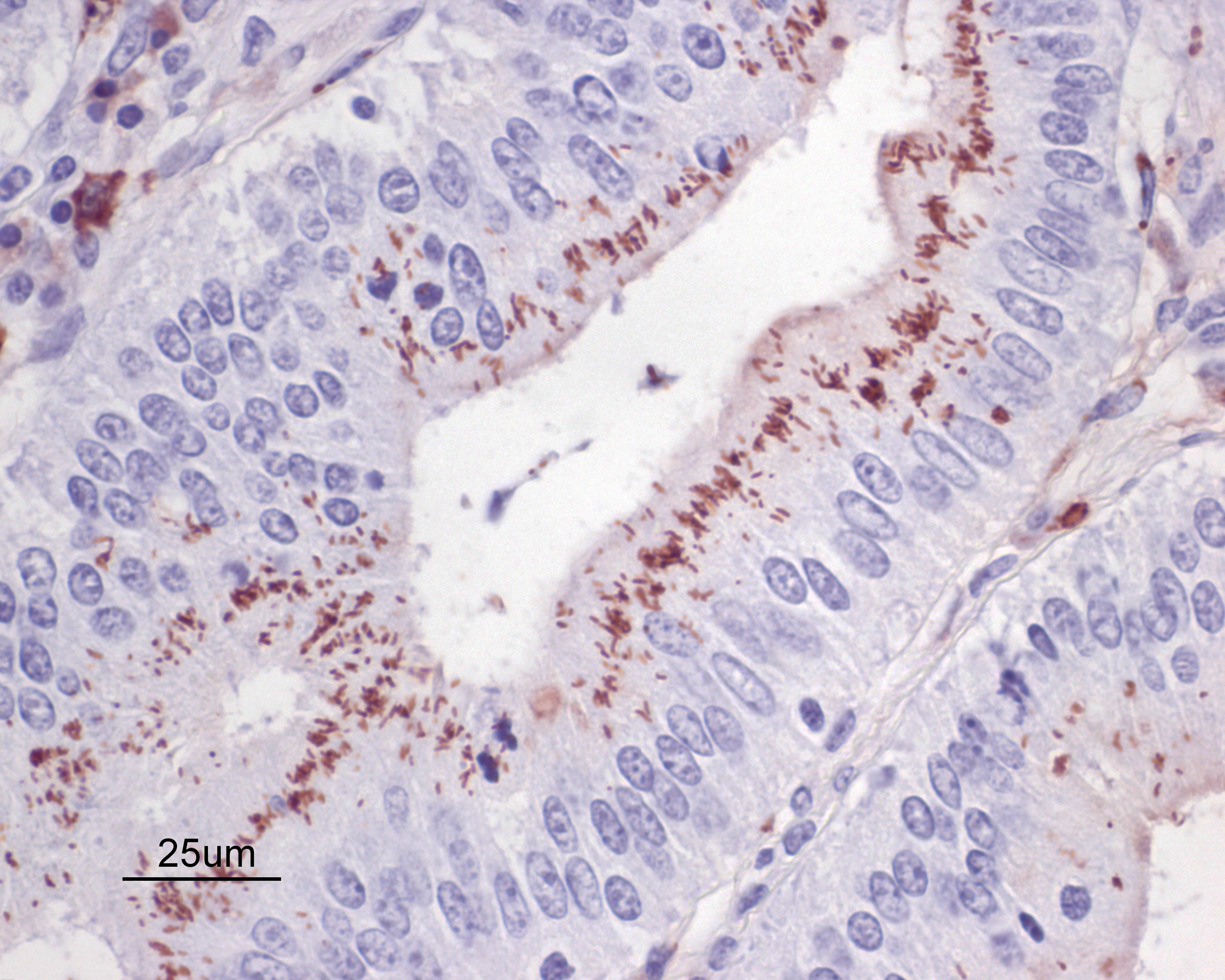
Warthin Starry silver stains of the intestine reveal numerous argyrophilic curved small (approximately 5 x 1 micron) bacilli concentrated in the apical cytoplasm of hyperplastic mucosal epithelial cells. Immunohistochemistry was performed using a polyclonal antibody to Lawsonia intracellularis, confirming the identity of the bacteria.
DNA was extracted from the frozen intestine and from formalin-fixed paraffin-embedded tissue scrolls. PCR was conducted to amplify short regions of the nuclear 18S rDNA (231 bp) and mitochondrial cytochrome c oxidase subunit I (COI) (512 bp). PCR and sequencing of the resulting amplicons identified the coccidia present as Eimeria furonis.
In addition, measurement of 6 oocysts observed in the intestinal content averaged 12.1 x 10.6 um with a shape index of 1.14. These measurements are also consistent with the dimensions of oocysts of E. furonis.
Contributor’s Morphologic Diagnoses:
Enteritis, proliferative, chronic, moderate with villus atrophy, crypt abscesses and numerous argyrophilic intraepithelial bacilli (Lawsonia intracellularis), and intralesional coccidia (Eimeria furonis)
Contributor’s Comment:
The debilitated state of this adult ferret is associated with concurrent infection by Lawsonia intracellularis and the coccidian parasite, Eimeria furonis.
The histologic lesions in this case are dominated by changes due to infection by Lawsonia intracellularis. L. intracellularis is a Gram-negative, non-spore-forming, microaerophilic, curved rod, and is an obligate intracellular pathogen (V). It is the etiologic agent of proliferative enteropathy (PE), characterized clinically by diarrhea associated with loss of functional mucosal surface area, as well as wasting due to protein-losing enteropathy. Histologically, PE is characterized by thickening of the distal small and/or large intestinal mucosa due to crypt enterocyte infection and proliferation. PE is an important endemic disease in swine herds and is also becoming an important disease of horses, predominantly weanling foals, worldwide. However, the bacterium can cause disease in a broad host range including donkeys, hamsters, rabbits, ferrets, foxes, dogs, rats, sheep, deer, emus, ostriches, nonhuman primates and guinea pigs.6 There are some differences in clinical and pathologic presentation of disease among affected species. Mucosal hyperplasia is characteristic of PE in all species, however the type and extent of associated inflammatory reactions vary and may depend on host-specific differences.6 The bacterium infects intestinal crypt cells, causing alterations in host gene expression resulting in induction of cell proliferation and mucosal thickening, as well as inhibition of secretory goblet cell and absorptive enterocyte differentiation leading to altered mucosal integrity.2 These changes are associated with simultaneous induction of Notch-1 signalling and attenuation of B-catenin/Wnt pathways.2
Several species of coccidia have been reported to infect the intestinal tract of the ferret, including Isospora laidlawi, I. eversmanni, Eimeria ictidea, E. furonis, and E. vision. Emeria hiepei also has been reported to infect the biliary epithelium. E. furonis is the most commonly identified species, and is generally associated with subclinical infections. E. furonis infects the small intestinal and rectal epithelium although there is a single case report of infection of the epithelium of hepatic bile ducts and gallbladder.5 Speciation of Eimeria is based on differentiating characteristics including host affected, specific location of the parasite within the host, and organism size and shape. This Eimeria species has approximately 12.8 x 12.0 um diameter, roughly spherical oocysts containing 4 sporocysts each with 2 sporozoites. By comparison, E. ictidea, a closely related species, has oocysts measuring 23 x 17.5 um. Laboratory identification can be assisted by sporulating viable oocysts from fresh feces in the laboratory, or using molecular techniques.1
Although historically rarely associated with clinical disease in ferrets, a recent report by Sledge et al. documents severe outbreaks of enteric coccidiosis due to E. furonis in three ferret rescue shelters, a situation similar to the case described here.5 Clinical signs in these groups of affected animals included foul smelling diarrheic feces, melena, lethargy, weight loss, dehydration, anorexia and weakness. In one of the reported outbreaks in a shelter population of 42 ferrets, greater than half were clinically affected and 7 died, while in a second group of 63 ferrets, 13 died and at least 21 additional animals displayed clinical signs but recovered. No other co-pathogens were discovered in these groups in this study. The authors suggest that high population density and the dynamic population structure of these groups, with regular introductions of newly rescued or adopted naïve animals may have predisposed to outbreak situations. Diagnosis can be challenging, as shedding of oocysts may be intermittent and may occur in low enough numbers to be not easily detected by routine fecal examination.
Contributing Institution:
Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada
http://ahl.uoguelph.ca
JPC Diagnosis:
- Colon: Colitis, proliferative, diffuse, severe with decreased goblet cells.
- Colon: Intraepithelial apicomplexan schizonts, gamonts, and oocysts, few.
JPC Comment:
Proliferative enteropathy is an economically important disease of swine production, with an estimated 96% of farms affected and a lost production cost of US $1 to $5 for every clinically affected pig.7 In swine, Lawsonia intracellularis infection can cause an acute hemorrhagic enteropathy; in chronic cases, it can cause proliferative intestinal adenomatosis or, in cases of secondary infection, can progress to necrotic enteritis.7 Pigs are infected through ingestion of the bacteria, which survive the acidic environment of the stomach and, once in the intestine, use polar flagella to reach the enterocytes.3, 7 The exact mechanism by which the bacteria enters enterocytes is unknown but may be mediated by a type III secretion system (T3SS).7 The bacteria proliferates by binary fission in the apical cytoplasm of infected cells.7 Spread of infection between enterocytes occurs mainly when infected progenitor cells giving rise to infected progeny; however, cell-rupture and liberation of bacteria can also lead to infection of neighboring enterocytes.3, 7
There are still many unknowns regarding pathogenesis of proliferative enteropathy, as the obligate intracellular and microaerophilic nature of L. intracellularis hinders research efforts.9 A recent study in yeast identified a potential effector protein, named LI1035, injected by the bacteria’s T3SS that may target the MAPK system and affect cell growth.9 Another study demonstrated that L. intracellularis can survive and possibly proliferate in porcine macrophages in vitro.3
A recent in vivo study in pigs uncovered the possible mechanisms behind mucosal hyperplasia and inhibition of goblet cell differentiation.2 As the contributor mentions, the cell signaling pathways β-catenin/WNT and Notch are complex intracellular mechanisms which control homeostasis and differentiation of enterocytes. Wnt-1 interaction with the membrane-bound Frizzled receptors prevents auto-phosphorylation of cytoplasmic β-catenin, which then migrates to the nucleus and induces gene expression associated with proliferation of intestinal stem cells, the precursor to transit-amplifying progenitor cells.2, 4 Separately, Notch receptor activation leads to translocation of the Notch receptor intracellular domain (NICD) into the nucleus, which stimulates gene expression that drives progenitor cell differentiation into an absorptive enterocyte and suppresses differentiation into the secretory phenotype (i.e. goblet cell).2 Huan et. al demonstrated that L. intracellularis infection was associated with increased levels of NICD1 and decreased levels of goblet-cell associated ATOH1 and MUC2. Their study also demonstrated increased cytomembranous (and thus decreased nuclear signaling) of β-catenin, which in mice induces proliferation of progenitor cells.2 This study provides a glimpse into the uncertain yet costly pathogenesis of L. intracellularis infections.
References:
- Abe N et al. First record of Eimeria furonis infection in a ferret, Japan, with notes on the usefulness of partial small subunit ribosomal RNA gene sequencing analysis for discriminating among Eimeria species. Parasitol Res 2008; 103:967-970.
- Huan YW, Bengtsson RJ, MacIntyre N, et al. Lawsonia intracellularis exploits β-catenin/Wnt and Notch signalling pathways during infection of intestinal crypt to alter cell homeostasis and promote cell proliferation. PLoS ONE. 2017;12(3):1-21.
- Pereira CER, Resende TP, Armien AG, et al. Survival of Lawsonia intracellularis in porcine peripheral blood monocyte-derived macrophages. PLoS ONE. 2020;15(7):1-10.
- Silva-Garcia O, Valdez-Alarcon JJ, Baizabal-Aguirre VM. Wnt/β-catenin Signaling as a Molecular Target by Pathogenic Bacteria. Frontiers in Immunology. 2019: 10(2135), 1-14.
- Sledge DG et al. Outbreaks of severe enteric disease associated with Eimeria furonis infection in ferrets (Mustela putorius furo) of 3 densely populated groups. J Am Vet Med Assoc 2011; 239: 1584-1588.
- Vannucci FA and Gebhart CJ. Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections. Vet Pathol 2014; 51:465-477.
- Vannucci FA, Gebhart CJ, McOrist S. Proliferative Enteropathy. In: Zimmerman JJ, Karriker LA, et al, eds. Diseases of Swine. 11th ed. Hoboken, NJ: John Wiley & Sons, Inc. 2019: 898-911.
- Williams BH, Chimes MJ, Gardiner CH. Biliary coccidiosis in a ferret (Mustela putorius furo). Vet Pathol 1996; 33: 437-439.
- Yang L Lai F, He L, et al. LI1035, a putative effector secreted by Lawsonia intracellularis, targets the MAPK pathway and regulates actin organization in yeast and mammalian cells. Veterinary Microbiology. 2019; 235:127-135.
- Gu J, Li X, Yang M, Du C, Cui Z, Gong P, Xia F, Song J, Zhang L, Li J, Yu C, Sun C, Feng X, Lei L, Han W. Therapeutic effect of Pseudomonas aeruginosa phage YH30 on mink hemorrhagic pneumonia. Vet Microbiol. 190:5-11, 2016.