Signalment:
Gross Description:
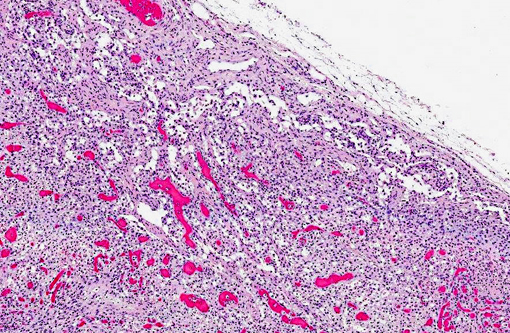
Histopathologic Description:
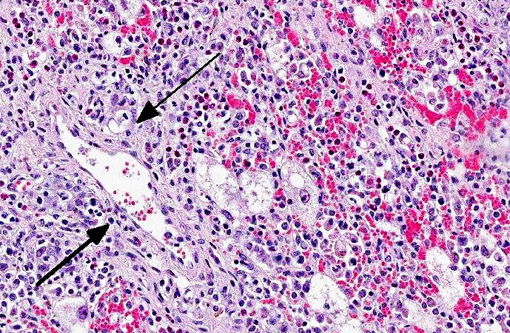
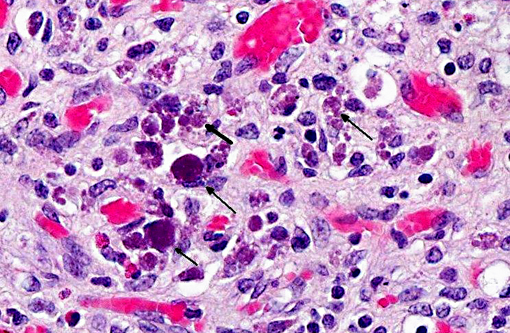
Mesenteric lymph node: Diffusely, there is severe lymphoid depletion with scattered karyorrhectic debris (necrosis). Also scattered throughout the section are large numbers of macrophages and eosinophils. The macrophages often contain botryoid basophilic glassy intracytoplasmic inclusion bodies. In fewer macrophages, intranuclear basophilic inclusions can be found.
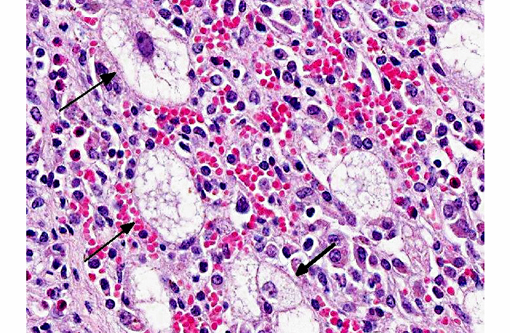
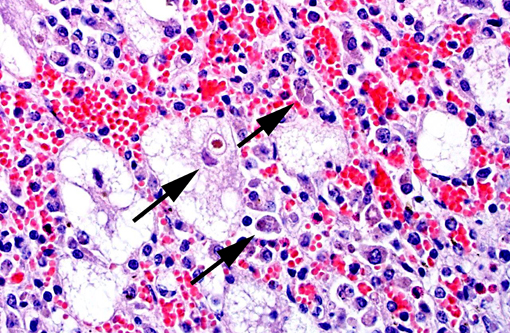
Liver: There is massive loss of hepatocytes, leaving disrupted liver lobules and dilated sinusoids engorged with erythrocytes. The remaining hepatocytes show severe swelling, with micro- and macrovesiculation of the cytoplasm and karyomegaly. Some swollen hepatocytes have basophilic intranuclear, irregular inclusions (degeneration). Throughout all parts of the liver there are scattered moderate to large numbers of macrophages (without inclusions). Within portal areas there is multifocally mild to moderate fibrosis and bile duct hyperplasia. Some bile duct epithelial cells show degeneration and necrosis, and there is infiltration of neutrophils within the lumen. The limiting plate is often obscured mainly by infiltrating macrophages and eosinophils, and fewer neutrophils, extending into the adjacent parenchyma. Scattered are small areas with extra medullary hematopoiesis.Â
Morphologic Diagnosis:
1. Mesenteric lymph node: Severe lymphoid depletion with moderate diffuse chronic granulomatous lymphadenitis, with intralesional botryoid basophilic intracytoplasmic and intranuclear inclusion bodies.
2. Liver: Severe hepatic degeneration and hepatocellular loss with severe diffuse chronic granulomatous hepatitis and mild neutrophilic cholangitis.
Lab Results:
Condition:
Contributor Comment:
As suggested by the different syndromes associated with PCV-2 infection, clinical signs can be variable. The typical clinical picture includes enlarged lymph nodes, decreased weight gain or wasting, combined with dyspnea, diarrhea, pallor or jaundice. Other signs that have been described include coughing, fever, central nervous system signs, and sudden death.(7)
In the case presented here, the liver lesions were the most striking feature. Although not as widely known as the respiratory, intestinal and cardiovascular lesions,(2) several reports have described lesions similar to those seen in this case. In a field study investigating 100 livers from pigs with clinical PCV2-associated disease, in 70% of the livers, the virus was associated with hepatocytes, Kupffer cells, and inflammatory infiltrates.(9) Hepatic lesions were reproduced experimentally in cesarean-derived colostrum-deprived and gnotobiotic pigs that were infected with PCV-2. Both moderate-to-severe necrotizing and granulomatous hepatitis, acute hepatitis with centrilobular necrosis of hepatocytes, and hepatic atrophy associated with nonsuppurative cholangiohepatitis were observed. In fetuses, the hepatic lesions were described as congestion with hepatocellular loss and nonsuppurative hepatitis with periacinar necrosis.(1,7)
The current case is a representation of the previously described end-stage hepatic disease(1) with extensive swelling and vacuolation of remaining hepatocytes with karyomegaly and progressive replacement of hepatocytes by histiocytic cells. Although immunohistochemistry was not available for this case, PCV-2 antigen is detectable early on in the disease process within the nuclei of hepatocytes and, as the disease progresses, within the cytoplasm of Kupffer cells and infiltrating mononuclear phagocytes.Â
The lymph node submitted together with the liver sample is a classical histologic presentation of the lymphadenopathy seen in the disease caused by PCV-2: severe depletion of lymphocytes and histiocytic infiltration of lymphoid tissues. It is thought that PCV-2 virus replicates within the histiocytes of lymph nodes. A study regarding the subcellular localization of PCV-2 virus found that in affected lymph nodes, viral particles were exclusively found in histiocytes. The ultrastructural changes found associated with the presence of viral particles were dilatation of the rough endoplasmic reticulum and swelling of mitochondria. With colocalization studies, a close relationship was found between the viral particles and the mitochondria, suggesting that these organelles play a role in replication of the virus.(8)
JPC Diagnosis:
1. Liver: Hepatitis, granulomatous and eosinophilic, diffuse, severe, with portal fibrosis, biliary ductal reaction, hepatocyte karyo/cytomegaly, vacuolar degeneration, chronic-active cholangitis, and rare intracytoplasmic viral inclusion bodies.
2. Lymph node: Lymphadenitis, granulomatous and eosinophilic, diffuse, chronic, severe, with lymphoid depletion and intrahistiocytic intracytoplasmic botryoid viral inclusion bodies.
Conference Comment:
PCVAD (formerly PMWS) involves multiple organ systems with a highly variable spectrum of gross lesions, the most common of which are emaciation, lymphadenopathy and mild interstitial pneumonia. Activation of the immune system followed by circovirus infection is required for the development of PMWS. Severely affected pigs may develop immunosuppression, which increases susceptibility to opportunistic infections and may result in a poor immune response to vaccines. Reported clinical signs include wasting, dyspnea, coughing, diarrhea, pallor, fever, central nervous signs, and sudden death. Although its name implies that clinical disease develops exclusively in recently-weaned pigs, PMWS can also affect mature pigs.(4,7,10)
PRDC is a multifactorial condition involving several coexisting etiologic agents, especially swine influenza, porcine respiratory and reproductive syndrome (PRRS) virus, PCV2, and porcine respiratory coronavirus. In addition to causing direct damage to the airway and lungs, these viruses predispose swine to secondary infection with pathogens such as Mycoplasma hyopneumoniae, Pneumocystis carinii, Pasteurella multocida, Streptococcus suis, Bordetella bronchiseptica and Haemophilus parasuis.(4) Typically, affected pigs are around 12 to 24 weeks of age and present with fever and varying degrees of sneezing, coughing, nasal discharge, and respiratory distress as well as reduced weight gain.(2,4,7)
PDNS is characterized by a systemic necrotizing vasculitis with tropism for kidney and skin, likely secondary to immune complex deposition. Although the development of PDNS has been attributed to PCV2, this condition has also been reproduced with pathogens other than PCV2, including PRRSV, Torque teno virus (arterivirus), Staphylococcus hyicus, Pasteurella multocida and Streptococcus sues. Grossly, affected swine exhibit irregular red to purple, often crusted papules over hindquarters, perineal area, and ears, in combination with bilaterally enlarged, pale kidneys with petechial hemorrhages.(3,5,7) PCV2 is not a primary cause of skin lesions but, in addition to the vasculitis and necrotizing skin lesions described in connection with PDNS, the virus has been demonstrated in combination with Staphylococcus hyicus in several cases of severe exudative epidermis.(7)
Reproductive failure due to PCV2 is characterized by stillbirths, mummification, embryonic death, and infertility (SMEDI). Reproductive failure is probably the least important manifestation of PCV2, as it is usually only seen in individual dams and is thus not of great economic importance.(7)
Regardless of the tissue or organ affected, PCV2 tends to produce similar histological lesions. Specifically, intracytoplasmic botryoid viral inclusions, granulomatous inflammation and necrotizing vasculitis are common microscopic findings associated with PCV2. Interestingly, there are typically no PCV2-induced microscopic lesions in the musculoskeletal, endocrine and reproductive (although as noted above, sporadic abortions are reported) systems.(2,4,7)
Although the specific mechanisms of PCV2 infection are not completely understood, recent studies have clarified several aspects of its pathogenesis. Viral antigen and/or nucleic acid may be found in multiple cell types; however, histiocytes are the main site of virus localization. Viral attachment to host cell surface receptors is mediated by heparan sulfate and chondroitin sulfate B on the viral surface.(6) Subsequently, histiocytic internalization of PCV2 occurs via clathrin-mediated endocytosis. Recent studies suggest that PCV2 replication also occurs within lymph node histiocytes and that the mitochondria likely play an important role.(8) Once internalized, PCV2 can transiently induce the PI3K/Akt pathway which inhibits apoptosis, thus promoting cell survival and viral replication. PI3K activates its downstream effector, the serine/threonine kinase Akt (also known as PKB), which phosphorylates various substrates, such as caspase-9, BAD, glycogen synthase kinase 3, and FKHR. Overall, this series of reactions leads to induction of NF-κβ and, ultimately, cell survival and growth, as well as the prevention of apoptosis via activation of antiapoptotic factors and inactivation of proapoptotic factors. Akt also results in activation of mTORC1 (which controls cell growth) and mTORC2 (which regulates the actin cytoskeleton), as well as JNK and p38 (which are involved in PCV2-induced apoptosis).(10)
References:
1. Allan GM, Ellis JA. Porcine circovirus: a review. J Vet Diagn Invest. 2000;12:3-14.
2. Caswell JL, Williams KJ. Porcine circovirus and postweaning multisystemic wasting syndrome. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol. 2. 5th ed. Philadelphia, PA: Elsevier Saunders; 2007:583-858.
3. Langohr IM, Stevenson GW, Nelson EA, et al. Vascular lesions in pigs experimentally infected with porcine circovirus type 2 serogroup B. Vet Pathol. 2010;47(1):140-147.
4. Lopez A. Respiratory system, mediastinum and pleurae. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:519-524.
5. Maxie MG, Newman SJ. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol. 2. 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:461-462.
6. Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J Virol. 2006;80(7):3487-3494.
7. Opriessnig T, Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol. 2013;50(1):23-38.
8. Rodriguez-Carino C, Sanchez-Chardi A, Segales J. Subcellular immunolocalization of Porcine Circovirus type 2 (PCV2) in lymph nodes from pigs with post-weaning multisystemic wasting syndrome (PMWS). J Comp Path. 2010;142:291-299.
9. Rosell C, Segales J, Domingo M. Hepatitis and staging of hepatic damage in pigs naturally infected with Porcine Circovirus type 2. Vet Pathol. 2000;37:687-692.
10. Wei L, Zhu S, Wang J, Liu J. Activation of the phosphatidylinositol 3-Kinase/Akts signaling pathway during porcine circovirus type 2 infection facilitates cell survival and viral replication. J Virol. 2012;86(24):13589-13597.