Signalment:
Young male York-shire/Hampshire
cross pig (
Sus domestica).Two
piglets (about three-weeks-old) had been growing slowly and showed respiratory
symptoms with rapid breathing. They were both treated with antibiotics
(penicillin) but died suddenly. The piglets were sent to necropsy. A few weeks
later a small number of fattening pigs had reduced growth rate and were in poor
body condition, the pigs were euthanized and sent to necropsy. All pigs were
from the same specific pathogen-free farm (SPF). The submitted slide is from
one of the fattening pigs.
Once a year tests are taken to show
freedom from: swine influenza virus,
Actinobacillus pleuropneumoniae
(APP),
Mycoplasma hyopneumoniae (SEP),
Sarcoptes scabiei, Swine
dysentery (
Brachyspira hyo-dysenteriae),
Intestinal spirochetosis (
Brachyspira pilosicoli), other
Brachyspira-subspecies, toxin-producing
Pasteurella multocida,
Salmonella
and
Lawsonia intracellularis. The farm was not vaccinated against
PCV-2.
Gross Description:
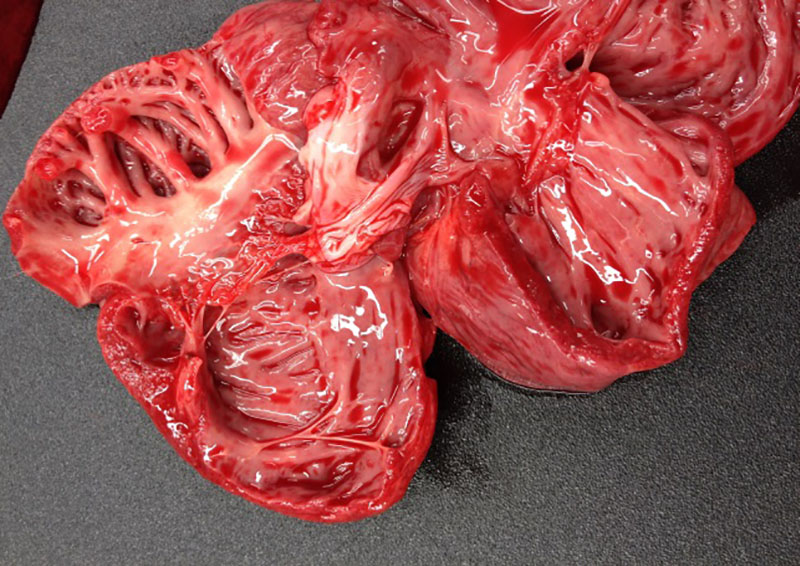
At
necropsy, the fattening pig was in poor body condition and presented with a
mottled myocardium, enlarged lymph nodes, multiple white foci scattered
throughout the kidneys, firm cranioventral lung, and mild ascites. The two
piglets were in poor body condition, the hearts had marked dilatation of left
and right ventricle and atrium (dilated cardio-myopathy) and the piglets
presented signs of cardiac failure with acute stasis in liver, ascites, and
pulmonary edema.
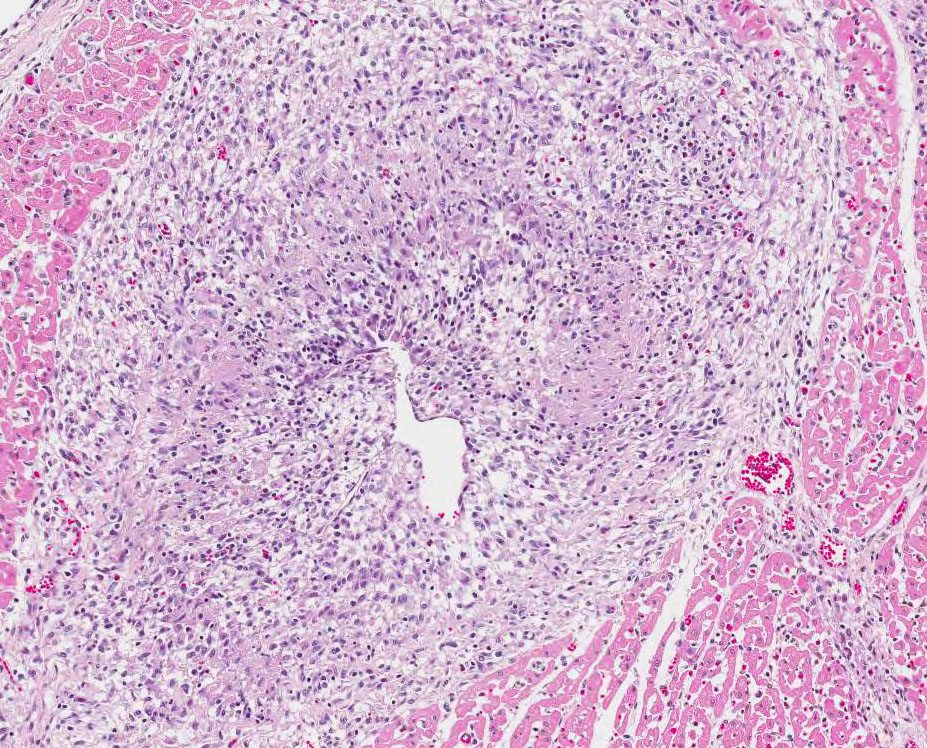
Histopathologic Description:
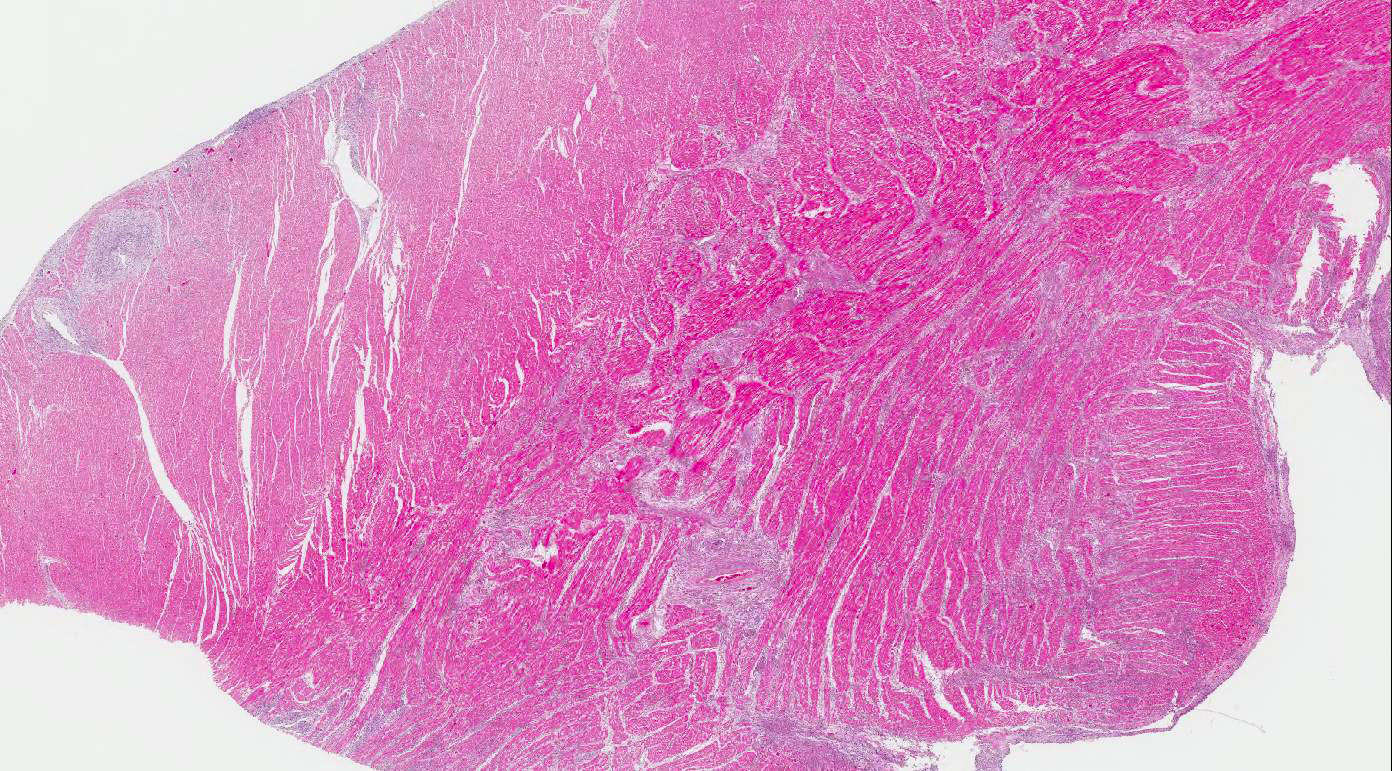
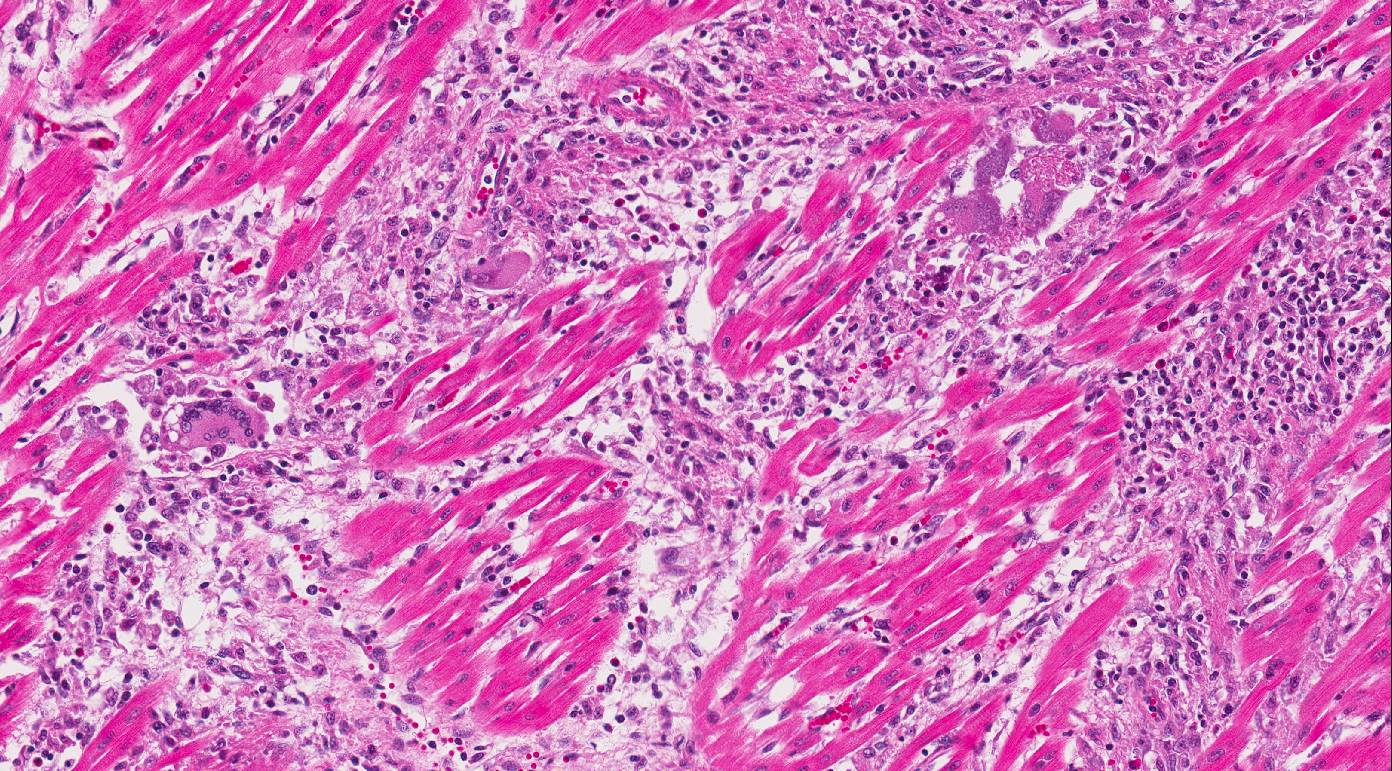
Heart:
Multifocal to coalescing chronic severe granulomatous interstitial inflammation
with loss of myocytes is present. The inflammatory cells are dominated by
lymphocytes, plasma cells, and macrophages with fewer neutrophils and
eosinophils. Few multinucleated giant cells are also seen. Multifocally, the
myocytes are degenerated or necrotic with loss of striations and eosinophilic
swollen cytoplasm, loss of nuclei, and occasionally pyknotic nuclei, scattered
degenerated Purkinje fibers are also seen. Multifocally, there are small foci
of basophilic homogenous material (mineralization). The outer walls of medium
and large sized vessels are infiltrated by varies amount of mononucleated
inflammatory cells and the vessels have marked thickened tunica adventitia due
to inflammation and edema, multifocal small sized vessels have degenerated
walls with infiltration of mixed inflammatory cells (vasculitis). The
endocardium is moderately thickened with abundant mixed inflammatory cells,
reactive fibroblasts and angiogenesis (granulation tissue). On
the Masson trichrome stain, there is a moderate amount of extracellular
collagen present in areas with myocyte loss and with reactive fibroblasts.
Morphologic Diagnosis:
Heart: Myocarditis and endocarditis,
multifocal to coalescing, interstitial, severe, chronic, lymphoplasmacytic and
histiocytic with multinucleated giant cells (granulomatous) and vasculitis.
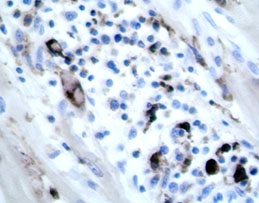
Lab Results:
Immunohistochemistry
heart: positive for PCV-2 (piglet). PCR lymph node: Positive for PCV 2 (fattening
pig).
Condition:
Myocarditis and endocarditis/PCV-2
Contributor Comment:
Porcine
circovirus (genus
Circovirus, family
Circoviridae) is a small
(17nm diameter), non-enveloped virus that contains circular single-stranded
DNA.
3,8 The virus was initially discovered in 1998 and was first
isolated from pigs with
post weaning multisystemic wasting syndrome
(PMWS). The virus is highly prevalent in the domestic pig population all over
the world and PCV-2 infection is common in herds as a subclinical disease.
PCV-2 infection has been associated with several disease complexes
including PMWS, enteric disease, respiratory disease, porcine dermatitis and
nephropathy syndrome and reproductive disorders.
3,8,10 Vaccine
against PCV-2 has been used since 2006, which have been effective controlling
and preventing PCV-2 associated diseases. Although the majority of the
conventional pigs entering the market are now vaccinated, PCV-2 still remains
an important differential diagnosis for various disease manifestations in pigs.
8
In PMWS, pigs exhibit a systemic infection
involving several organ systems and the disease is characterized by loss of
weight or wasting in combination with various other clinical signs such as
dyspnea, diarrhea, pallor, and jaundice. Other clinical signs include coughing,
fever, central nervous signs, or sudden death.
3,8 On gross necropsy,
lymphadenopathy is the most consistent feature of PMWS, but the gross lesions
and severity are highly variable.
8 Diagnosis is mainly based on
clinical findings and typical gross and histological lesions, and is supported by
demonstration of active PCV2 infection by immuno-histochemistry or PCR.
3
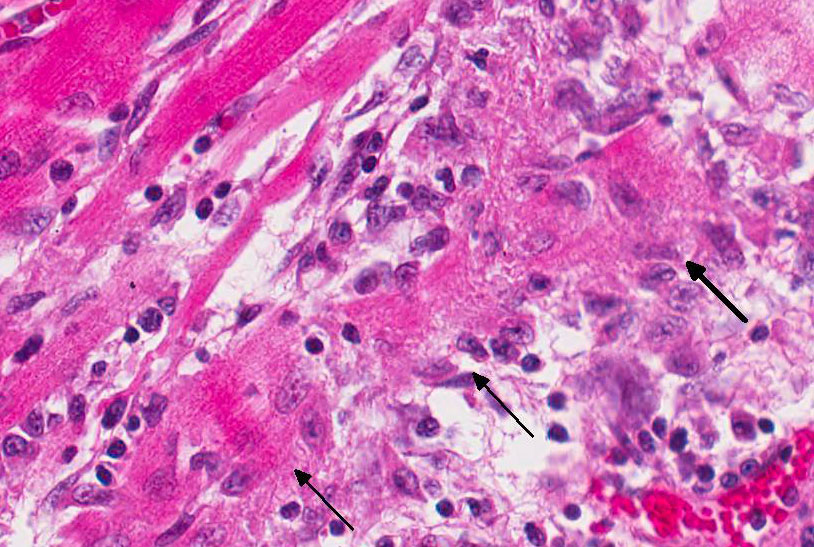
Porcine
circoviral antigen and nucleic acid are most consistently present in the
cytoplasm of monocytes, macrophages and dendritic cells throughout the body.
Vascular smooth muscle and endothelial cells occasionally express circoviral
antigen.
3 PCV2 induces characteristic lesions in the lymphoid system
which includes lymphoid depletion and granulo-matous inflammation with multi-nucleated
giant cells, lesions are commonly observed in the tonsils, spleen, Peyer´s
patches, and lymph nodes. In the respiratory system, bronchinterstitial
pneumonia may be a feature and the virus can cause meningoencephalitis and
vasculitis in CNS. In the digestive system, the most frequent lesion is
granulomatous enteritis characterized by increased number of macrophages and
scattered multinucleated giant cells in the mucosa and submucosa of the ileum
and occasionally the colon and cecum. In the kidney, lesions are characterized
by lymphoplasmacytic or granulomatous interstitial nephritis. In the vascular
system, PCV2 antigen has been demonstrated in endothelial cells and in
inflammatory cells in the arterial walls and chronic lymphoplasmacytic vascular
lesions has been described among pigs infected with PCV2.
8,10 In
foetuses, lesions are most prominent in the cardiovascular system, especially
in the heart where cardio-myocytes are degenerated, or lost and replaced by
fibrous connective tissue. In addition, there is non-suppurative myo-carditis
and occasionally multinucleated giant cells.
2,6, 8,11 Heart failure
and dilated cardiomyopathy is described as a feature among aborted foetuses and
very young animals secondary to myocarditis.
2,6,8,11
The presented case from the pig-farm
showed typical gross and histological lesions of PCV2 infection that are
compatible with the described cases in the literature. The fattening pig had
lymphoid depletion and granulomatous inflammation in lymph node and granulomatous
inflammation with vasculitis in kidney and heart. A purulent bronchopneumonia
was seen in the lungs; however, the material was not cultured but secondary
bacterial infection is most likely. The two young piglets with dilated
cardiomyopathy had chronic granulomatous myocarditis at on histopathology. The
diagnosis was supported by demonstrating active PCV2 infection with PCR and immuno-histochemistry.
JPC Diagnosis:
Heart:
Pancarditis, transmural, lymphohistiocytic, multifocal to coalescing, marked,
with vasculitis, myocardial loss, and fibrosis, York-shire/Hampshire cross pig,
Sus
domestica.
Conference Comment:
The contributor provides an excellent summary of porcine circovirus-2 (PCV-2)
infection in swine. There are two separate genotypes of PCV have been
implicated in this species. PCV type 1 (PCV-1) is generally thought to be
nonpathogenic and does not cause disease in pigs, although it has been
implicated as a potential cause of congenital tremors in newborn piglets.
3
PCV-2 is pathogenic in pigs and causes post weaning multisystemic wasting
syndrome (PMWS) discussed by the contributor above. PMWS is a multifactorial
systemic disease and clinically manifests at 25-150 days of age with most cases
occurring between 7 and 15 weeks.
3-6,9 The six fundamental clinical
signs include wasting, dyspnea, lymphadenopathy, diarrhea, pallor, and
jaundice. Coughing, fever, gastric ulceration, and meningitis have also been
reported. Serum antibodies to PCV-2 are very common in swine herds around the
world, and positive antibody titer does not necessarily equate to clinical
PMWS.
3 However, co-infection of PCV-2 with porcine reproductive and
respiratory syndrome virus (PRRSV), and/or porcine parvovirus (PPV), produces
more severe clinical disease; although, PCV-2 has been shown to be sufficient
to produce disease in susceptible animals without co-infection.
3,4,6,9,10
Conference participants
discussed that lymphoplasmacytic and histiocytic myo-carditis with varied
numbers of multi-nucleated giant cells and myocardial degeneration, loss, and
replacement by fibrosis are typical histologic changes associated with
infection by PCV-2.
3,4 In addition, necrotizing vasculitis, a key
histologic feature in this case, has been implicated as a hallmark lesion in
PCV-2 infection, as well as the severe form of systemic porcine
circovirus-associated disease (PCVAD), discussed below.
1-3,9
Participants also noted the lack of unique and characteristic intracytoplasmic
baso-philic botryoid viral inclusion bodies, typically associated with PCV-2
infection. Inclusion bodies may be present within macrophages;
however, they are not always identified. Definitive diagnosis of PMWS is based
on a combination of typical clinical signs of disease, typical histologic
lesions, and detection of PCV-2 in affected tissues via immunohistochemistry,
as demonstrated by the contributor in this case.
3
As mentioned above, infection with PCV-2 alone can cause disease in pigs;
however, it is more commonly identified in a complex of multiple pathogen
infection, known as PCVAD. This syndrome results from co-infection of PCV-2
with PPV, PRRSV, encephalomyocarditis virus (EMCV), swine influenza virus, or
Mycoplasma
hyo-pneumonia, or a
combination of these agents.
1,3,4,10 It can also occur from PCV-2
infection in association with recent vaccination.
3,4 Despite the
economic losses caused by PCV-2, PMWS, and PCVAD, the pathogeneses underlying
the clinical findings remain largely unclear; however, they are likely related
to macrophage activation prior to infection and subsequent endothelial cell
modulation.
7 Conference participants discussed that antigens for
PCV-2 can be found in macrophages, monocytes, and dendritic cells, as well as
endothelial cells and vascular smooth muscle.
References:
1. Allan GM, Ellis
JA. Porcine circoviruses: A review. J Vet Diagn Invest. 2000; 12:3-14.
2. Brunborg IM,
Jonassen CM, Mildal T, et al. Association of myocarditis with viral
load of porcine circovirus type 2 in several tissues in cases of foetal death
and high mortality in piglets. A case study.
J Vet Diagn Invest 2007;
19:368-375
3. Caswell JL and
Williams KJ. Respiratory system. In: Maxie MG, ed.
Jubb, Kennedy and
Palmer´s. Pathology of Domestic Animals 6th ed Vol. 2. St Louis, MO:
Elsevier Saunders; 2016:527-529.
4. Cushing TL,
Steffen D, Duhamel GE. Pathology in practice: Myocarditis attributable to PCV-2
infection in a pig fetus. J Am Vet Med Assoc. 2013; 242:317-319.
5. Harding JC. The clinical expression and emergence of porcine
circovirus 2.
Vet Microbiol. 2004; 98:131-135.
6. Madson
DM, Patterson AR, Ramamoorthy S, et al. Effect of Porcine Circovirus Type 2
(PCV2) vaccination of the dam on PCV2 Replication in utero.
Clinical and
vaccine immunology.
2009; 830-834.
7. Marks
FS, Almeida LL, et al. Porcine circovirus 2 (PCV2) increases the expression of
endothelial adhesion/junctional molecules.
Braz J Microbiol. 2016;
47:870-875.
8. Opressnig
T, Janke BH and Halbur PG. Cardiovascular lesions in pigs naturally or
experimentally Infected with porcine circovirus type 2.
J Comp. Path. 2006;
134:104-110.
9. Opriessnig
T, Langohr I. Current state of knowledge on porcine circovirus type
2-associated lesions.
Vet Pathol. 2012; 50(1):23-33.
10. Segalés J.
Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and
laboratory signs.
Virus Res. 2012; 164:10-19.
11. West KH, Bystrom
JM, Wojnarowicz C, Shantz N, et al. Myocarditis and abortion associated with
intrauterine infection of sows with porcine circovirus 2.
J Vet Diagn Invest.
1999; 11:530-532