Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 17
January 31st, 2018
CASE III: S-13-1538 (JPC 4085535).
Signalment: 2-year-old, female, Aberdeen Angus, Bos taurus, bovine.
History: Seventy, 1.5- to 2-year-old Aberdeen Angus, Hereford and mixed breed steers and heifers were grazing a mixed pasture composed of Festuca arundinacea, Lotus corniculatus, and Trifolium repens and supplemented with Trifolium pretense haylage. The herd was managed in a rotational grazing system, and paddocks were sprayed with a commercial liquid formula for bloat prevention. The animals had access to an old abandoned building lodging waste material, including an unlabeled bucket containing an unidentified gray-white powder. Over a period of 21 days, 14 animals (20%) became sick, 10 (14%) died, and 4 recovered. Affected animals had diarrhea, melena, mild ataxia, and/or difficulty standing. The clinical course in fatal cases was 12-18 hrs.
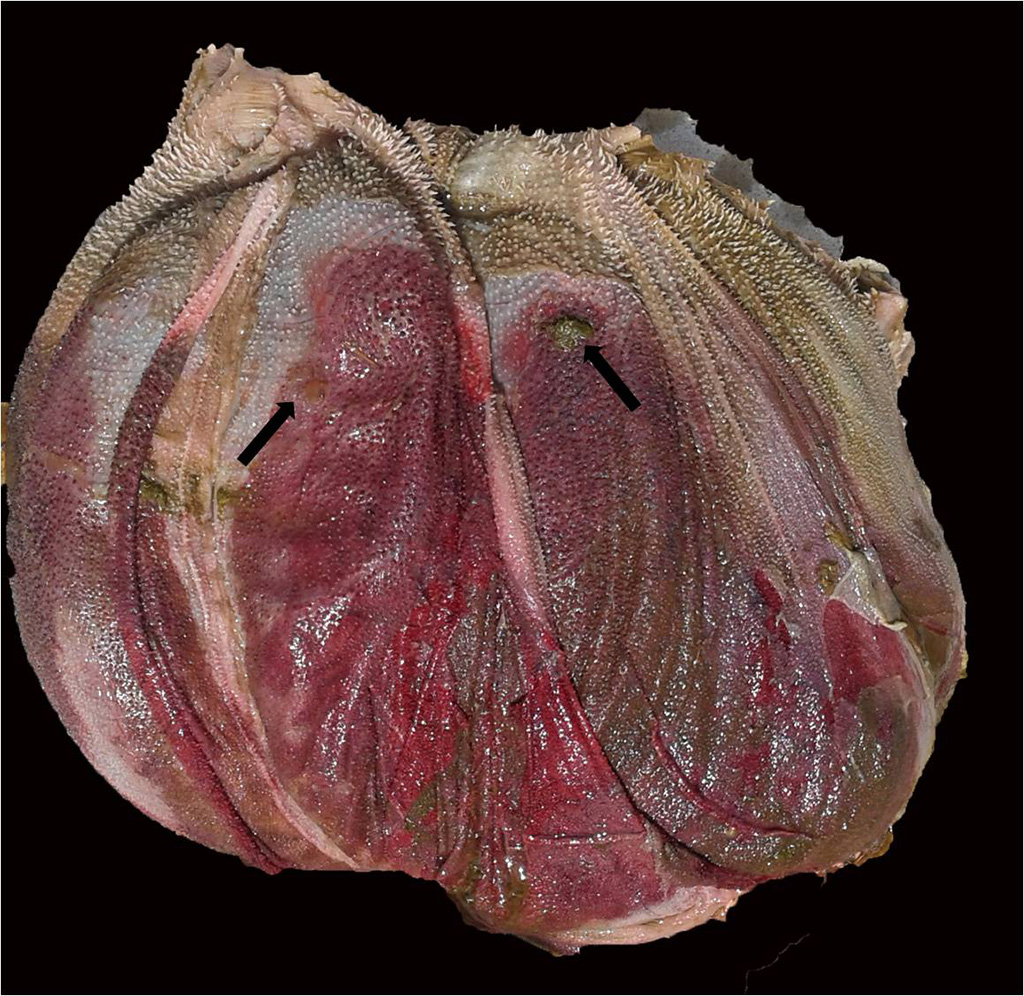
Gross Pathology: Three cattle were autopsied. The main gross findings included severe diffuse congestion and hemorrhage of the mucosa of the abomasum, omasum and rumen, with multifocal extensive mucosal/epithelial sloughing, erosions and ulcers in the forestomachs. The submucosa of the abomasum and forestomachs was moderately expanded by red-tinged gelatinous material (edema and hemorrhage). In one of the animals there was diffuse bilateral pallor of the cortical renal parenchyma.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
The arsenic concentration (DM) in the liver of three animals were 20, 31 and 24 ppm, and lead concentrations were 8.3, 25 and 9.4 ppm, respectively. Mercury was no detected in any case.
Microscopic Description:
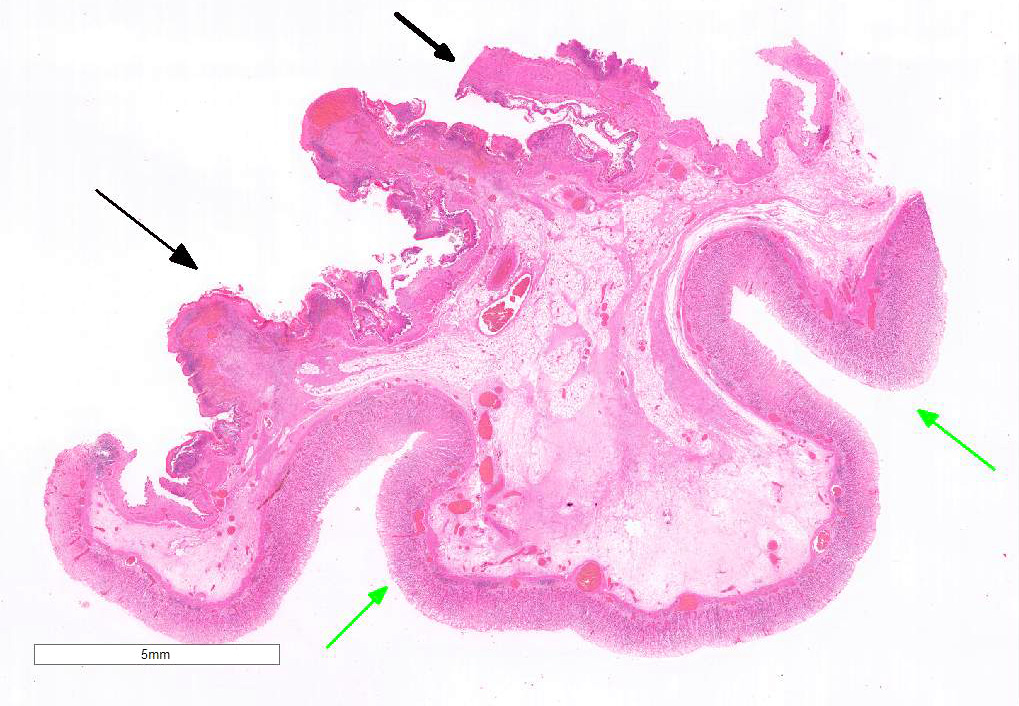
Examined is a section of the vela abomasica (omaso-abomasal ostium).
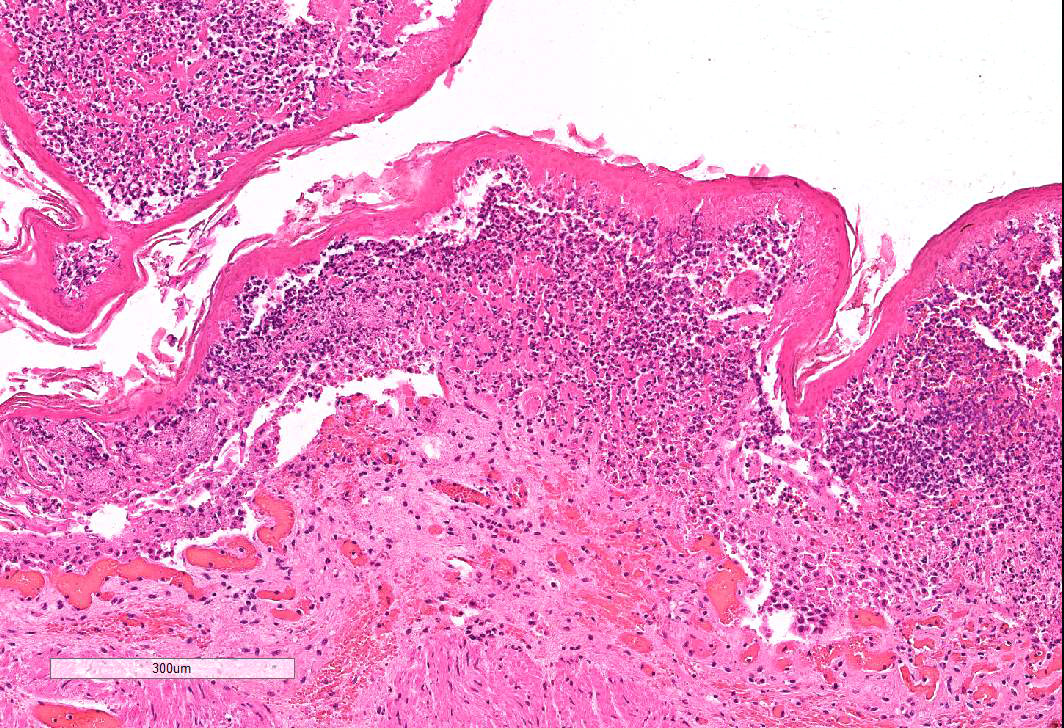
Omasum: Frequently, the mucosa is ulcerated with abundant necrotic cells, debris, fibrin, edema, hemorrhage, plant material and mixed bacterial colonies. Multifocally in more preserved areas, where the aforementioned changes are more superficial and do not reach the mucosal basement membrane, the basal layer of the epithelium is detached, and the basal cells are swollen and have a clear eosinophilic vacuolated cytoplasm, or exhibit individual cells necrosis. Multifocally, there are large intraepithelial spaces filled with abundant neutrophils, edema and fibrin (pustules). The submucosa underlying the ulcerated areas is multifocally expanded by edema and fibrin, extravasated erythrocytes, and viable and degenerate neutrophils. The submucosal blood vessels are either congested and lined by hypertrophic endothelial cells, or occluded by fibrin thrombi and lined by endothelial necrotic cells. The tunica muscularis and adventitia are variably expanded by abundant edema and moderate numbers of viable and degenerate neutrophils. Lymphatic vessels are multifocally ectatic.
Abomasum: Diffusely, mucosal and submucosal vessels are congested and there is multifocal extravasation of erythrocytes. Edema and few infiltrating eosinophils, neutrophils and lymphocytes are observed in the lamina propria. Some scattered parietal and chief cells exhibit degeneration or necrosis. Occasionally, glands in the deeper aspect of the mucosa are mildly ectatic, lined by attenuated epithelium and filled with small amounts of sloughed necrotic epithelial cells. The submucosa is diffusely expanded by edema and neutrophils. Note: in most sections there is variable autolysis in the mucosa of the abomasum.
Contributor’s Morphologic Diagnosis:
- Omasum: omasitis, necrohemorrhagic, suppurative, ulcerative, diffuse, severe, acute.
- Abomasum: abomasitis, necrohemorrhagic, diffuse, severe, acute.
Contributor’s Comment: The disease was diagnosed as arsenic poisoning. The results indicated arsenic intoxication and lead exposure. A sample of the powder found in the buckets in the abandoned building present in the paddock was submitted to a laboratory for determination of its chemical composition, and proved to be lead arsenate, an inorganic insecticide that used to be extensively used in agriculture.5 Retrospectively, it was found out that the farm where this outbreak occurred used to be an orange orchard a few decades ago. The current property owner had purchased the farm in recent years, and was unaware of the presence of this insecticide in this building. In several occasions the farmer had seen cattle entering the abandoned building where the insecticide had been inadvertently stored.
Arsenic is a ubiquitous toxic element that is concentrated in the environment as a result of industrial activities. Before the 1960s, arsenic was used extensively in pesticides, herbicides, fungicides, paint, and leather and wood preservatives.2,4 Arsenic has 3 oxidative stages, and intoxication is associated with either the trivalent (arsenite) or pentavalent (arsenate) forms. The pentavalent form is reduced to the trivalent form in the rumen. In cattle, the lethal dose 50% after ingestion is 1-25 mg/kg for trivalent arsenic, and 30-100 mg/kg for the pentavalent form.2
Although acute arsenic poisoning is uncommon nowadays, it should be considered in some particular epidemiological conditions that allow animals to access areas where the elements persist.2,6 Arsenic poisoning is due to sulfhydryl group binding, and inhibition of cellular metabolic activity.3 Arsenic is also a low-grade corrosive and irritant. Signs and lesions of acute arsenic poisoning in domestic animals are referable to the gastrointestinal tract (congestion, edema, hemorrhage, necrosis), liver (hepatocellular necrosis), and kidney (tubular necrosis). Skin and nervous lesions and signs occur in subacute to chronic poisoning, and include edema and petechiation of the brain (mediated by vascular injury), and dermatitis.2-6 In addition to the alimentary tract lesions presented in this conference, the microscopic examination of the kidneys in two of the examined cattle revealed diffuse nephrosis, which was supportive of systemic arsenic poisoning.
The main differential diagnosis for necrohemorrhagic and ulcerative lesions of the forestomachs in cattle in Uruguay is Baccharis coridifolia poisoning, which causes necrohemorrhagic lesions in the digestive tract; however no toxic plants were found in the pasture.1 Other differential diagnoses include viral agents such as bovine viral diarrhea virus (BVDV, mucosal disease), bovine herpesvirus-1 (BHV-1), malignant catarrhal fever (MCF) virus, and bluetongue disease virus. BVDV and BHV-1 infection were ruled out by immunohistochemistry in the omasum in this case.
JPC Diagnosis: Omasum: Omasitis, necrotizing, diffuse, severe with marked submucosal edema, Aberdeen Angus (Bos taurus), bovine.
Conference Comment: Arsenic poisoning may occur orally or percutaneously, with the percutaneous route having a lower toxic dose which is potentiated by high body temperature. Common sources of arsenic for animals include insecticides and herbicides, more specifically sodium arsenite, lead arsenate (the agent in this case), and arsenic pentoxide. The most susceptible organs are the brain, lungs, liver, kidney, and alimentary mucosa due to their high metabolic requirements. In general, the pattern of lesions corresponds to the route of intoxication except in pigs where the signs are only referable to the nervous system.3
There are two types of arsenicals, inorganic and organic, which each have trivalent and pentavalent forms. Of the two, inorganic arsenite (As3+) is the most toxic, followed by inorganic arsenate (As5+), trivalent organics, and pentavalent organics. In general, inorganics and trivalent organics affect the alimentary tract and vasculature, and pentavalent organics lead to neurologic signs. The main mechanism of action of arsenic is combination with an inactivation of sulfhydryl groups which results in systemically decreased metabolic activity. The clinical signs for inorganic arsenite (As3+), inorganic arsenate (As5+), and trivalent organics are similar. In the peracute form, very large amounts can result in sudden death within 24 hours with no gross lesions. In the acute form, less poison is ingested and there are several days after ingestion and before onset of clinical signs which include: vomiting, colic, weakness, staggering, ataxia, recumbency, watery diarrhea, rumen and gastrointestinal atony, shock, collapse, and eventually death. Microscopically, the lesions associated with acute toxicity can be attributed to vascular injury with marked gastric and intestinal mucosal and submucosal congestion, edema, hemorrhage, and ulceration. Liver and kidney are affected too with multifocal hepatic and renal proximal tubular necrosis. The subacute and chronic forms have the same distribution as the acute form with more severe clinical signs (fatigue, intense thirst, brick-red mucus membranes, and swollen joints) and, microscopically, glomerular changes which include capillary dilation which results in ischemia, proteinuria, and tubular necrosis eventually culminating in tubular fibrosis. In pigs specifically, organoarsenical phenylarsonic acid derivatives (arsanilic acid or 3-nitro-4-hydroxyphenylarsonic acid) are frequently used as feed additives to encourage growth and control of intestinal disease (ie. Swine dysentery). Toxic doses of either results in a spectrum of lesions from white matter edema in the central nervous system to Wallerian degeneration of the peripheral nerves.3
The differentials discussed above by the contributor can be ruled out based on the location of gross lesions and microscopic appearance. For instance, malignant catarrhal fever (bovine gammaherpesvirus) generally has ocular and oral lesions, and microscopically there will be perivascular lymphoproliferative lesions of predominately CD8+ T-cells. Bovine herpesvirus-1, or bovine rhinotracheitis, would have lesions in the mouth, pharynx, and trachea with eosinophilic intranuclear inclusion bodies microscopically. Finally, the mucosal disease form of bovine pestivirus and bluetongue (orbivirus) generally produce oral and nasal mucosal ulceration also.3
Conference attendees carefully considered the contributor’s morphologic diagnosis of necrohemorrhagic abomasitis; however the absence of hemorrhage or inflammatory infiltrates (other than aggregates of lymphocytes and plasma cells in the deep mucosa) or underlying vascular changes led to a unanimous consensus that the abomasal mucosal changes were the result of autolysis.
Contributing Institution:Plataforma de Investigación en Salud Animal
Instituto Nacional de Investigación Agropecuaria (INIA), Uruguay
www.inia.uy
References:
- Barros CSL. Livestock poisoning by Bacharis coridifoia. In: Garland T, Barr AC (ed).Toxic Plants and other Natural Toxicants. New York, NY: CAB International; 1998:569-572.
- Bertin FR, Baseler LJ, Wilson CR, Kritchevsky JE, Tylor SD. Arsenic toxicosis in cattle: meta-analysis of 156 cases. J Vet Intern Med. 2013; 27: 977–981.
- Cantile C, Youssef S. Nervous system. In: Maxie, MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol. 1. 6th Philadelphia, PA: Elsevier; 2015:327-328.
- Neiger R, Nelson N, Miskimins D, Caster J, Caster L. Bovine arsenic toxicosis. J Vet Diagn Invest. 2004;16:436–438.
- Peryea FJ. Historical use of lead arsenate insecticides, resulting soil contamination and implications for soil remediation. Proceedings of the 16th World Congress of Soil Science, Montpellier, France. 1998. Available at: soils.tfrec.wsu.edu/leadhistory.
- Selby LA, Case AA, Osweiler GD, Hayes HM Jr. Epidemiology and toxicology of arsenic poisoning in domestic animals. Environ Health Perspect. 1977; 19:183–189.