Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 23
24 April, 2019
CASE I: 12-324-3 (JPC 4032713)
Signalment: 6-year-old, spayed female, Labrador Retriever cross, dog, Canis familiaris
History: The dog presented to the referring veterinarian with a 3-day history of inappetance, vomiting, diarrhea, and lethargy. Radiography revealed an enlarged left kidney. A nephrectomy was performed and the dog recovered uneventfully. The following day the dog became lethargic and then died.
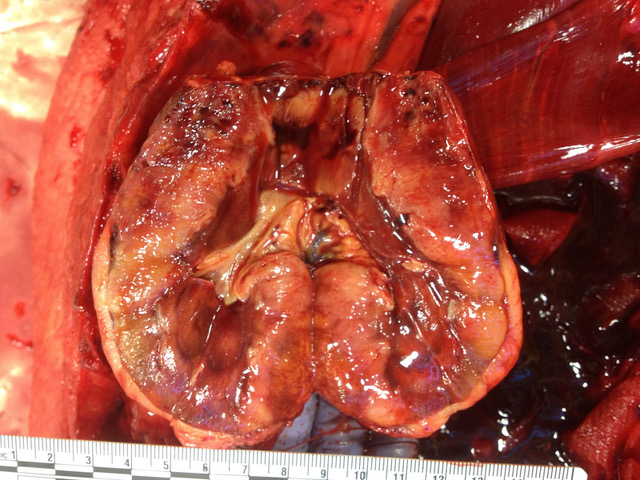
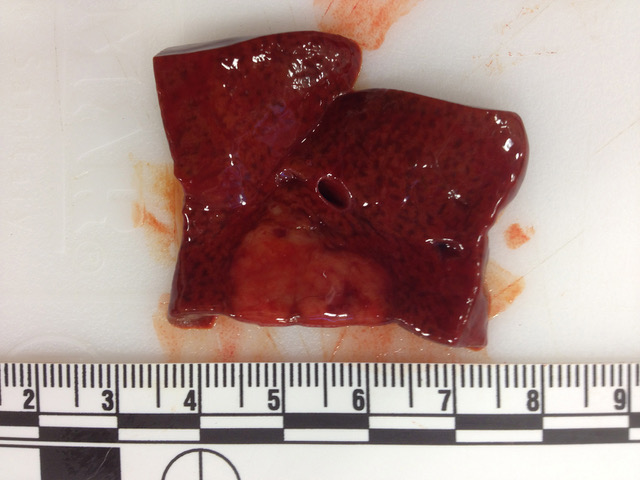
Gross Pathology: The carcass and nephrectomized left kidney were submitted for gross examination. Necropsy examination revealed severe hemoperitoneum, and death was attributed to exsanguination from the surgical excision site. Both kidneys were markedly enlarged and had irregular contours. On section the cortex and medulla were irregularly expanded by discrete to coalescing white patches and streaks. The liver contained approximately a dozen firm white parenchymal nodules up to 1 cm in diameter and had a diffusely accentuated lobular pattern. The mesenteric, renal and thoracic lymph nodes were moderately enlarged and, on section, lacked typical corticomedullary architecture.
Laboratory results: NA
Microscopic Description:
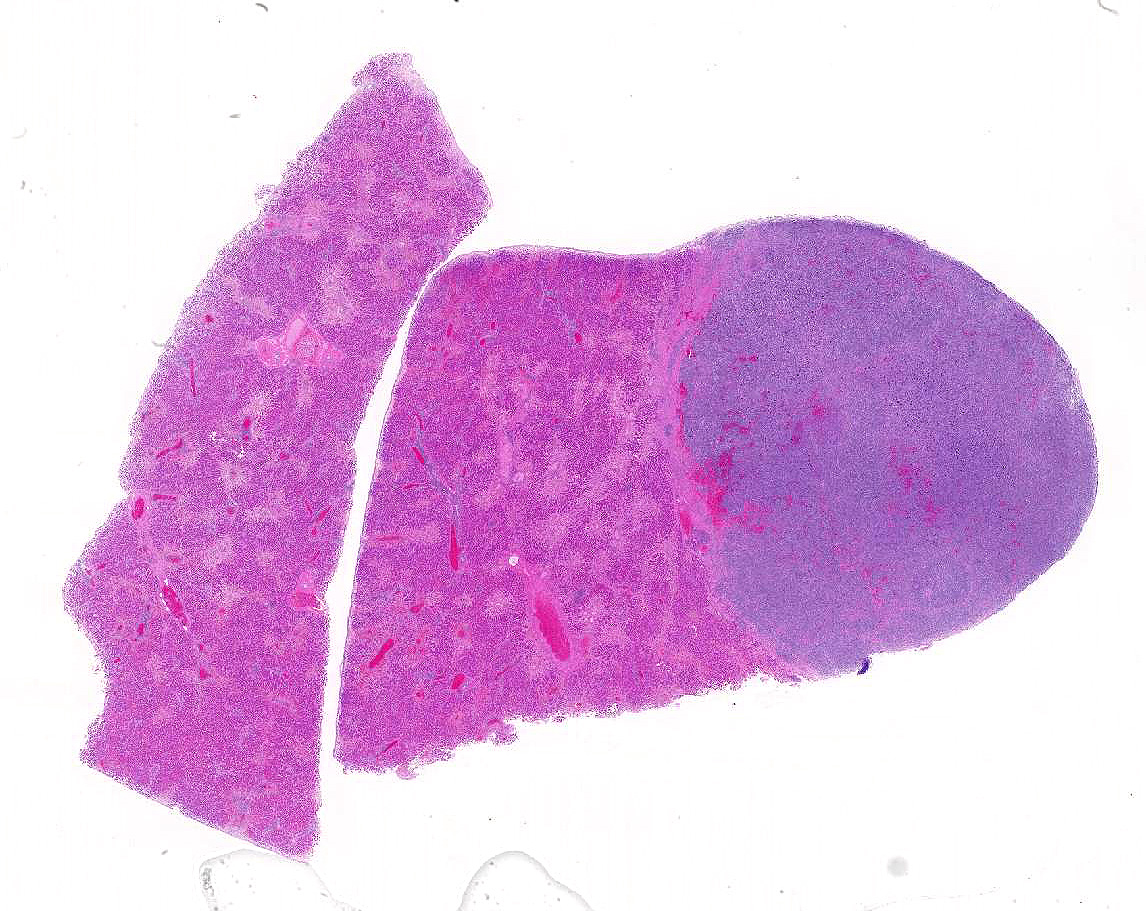
Liver: Two sections of liver are examined. One contains a 9mm diameter nodule (described in the gross pathology section above), and the other is from an unaffected part of the liver.
The nodule is unencapsulated but well circumscribed, slightly infiltrative at its edge and surrounded by a rim of compressed hepatic parenchyma. It consists of sheets of neoplastic cells forming two distinct populations of (a) small round cells and (b) larger, highly pleomorphic cells.
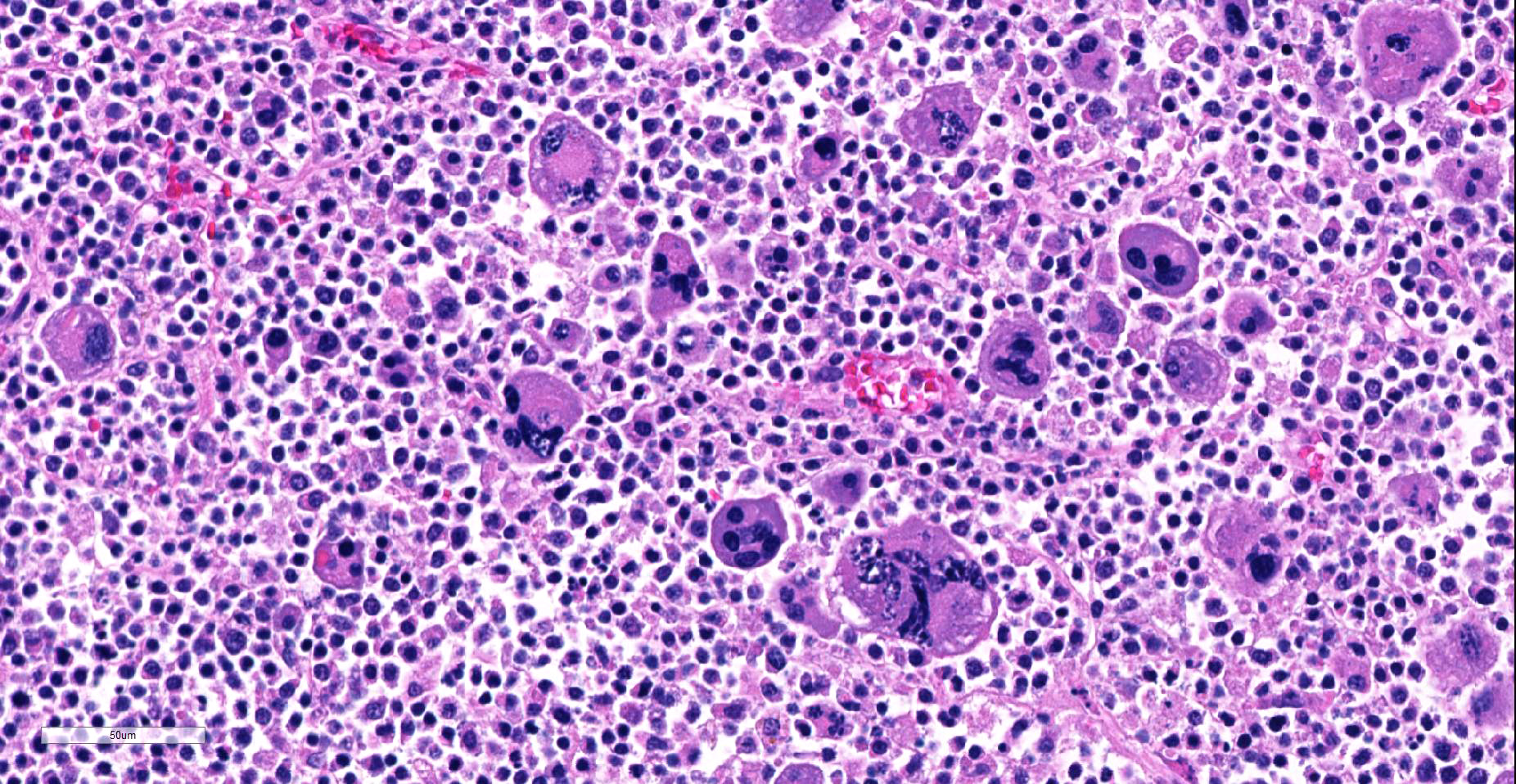
The first cell population makes up approximately 80-90% of the mass. Cells are small (8-15 µm diameter) and round with centrally to eccentrically placed nuclei and a scant to moderate amount of finely granular to homogeneous eosinophilic cytoplasm. Nuclei are round or slightly indented and mildly anisokaryotic with coarsely clumped chromatin and mainly inconspicuous nucleoli. In 10 HPF there are 4 mitotic figures.
The second population of neoplastic cells consists of several hundred highly pleomorphic cells from 15-150 µm in diameter scattered randomly among the smaller cells described above. The most prominent are giant cells with abundant amphophilic to eosinophilic cytoplasm, from 1 to 10 markedly anisokaryotic nuclei, and bizarre nuclear shapes and arrangements. However, numerous forms of these cells intermediate in size or similar in size to the small round cells described above are present. Larger cells often contain variably-sized, brightly eosinophilic, smudged cytoplasmic vacuoles.
Throughout remaining (non-nodular) hepatic parenchyma most portal tracts are heavily infiltrated by small round cells similar to those comprising the majority of the nodule described above. Centrilobular sinusoids and central veins are severely congested and centrilobular hepatocytes are atrophied or swollen with foamy cytoplasm (lipid accumulation). Kupffer cells near central veins contain abundant finely granular yellow brown pigment. Multifocally, adjacent centrilobular hepatocytes are outlined by thin, branching green-yellow lines (canalicular cholestasis).
Contributor’s Morphologic Diagnoses:
Liver: 1. B cell lymphoma; 2. Megakaryocytic neoplasia
Contributor’s Comment: Gross necropsy findings of bilateral irregular renomegaly, multiple pale hepatic nodules, and multifocal abdominal lymphadenomegaly led to a presumptive diagnosis of lymphoma. Histologically, hepatic portal infiltration by sheets of round cells supported this diagnosis. However, in addition to the population of presumed lymphocytes, bizarre giant and blastic cells were seen in solid hepatic masses, throughout both kidneys and throughout enlarged renal lymph nodes.
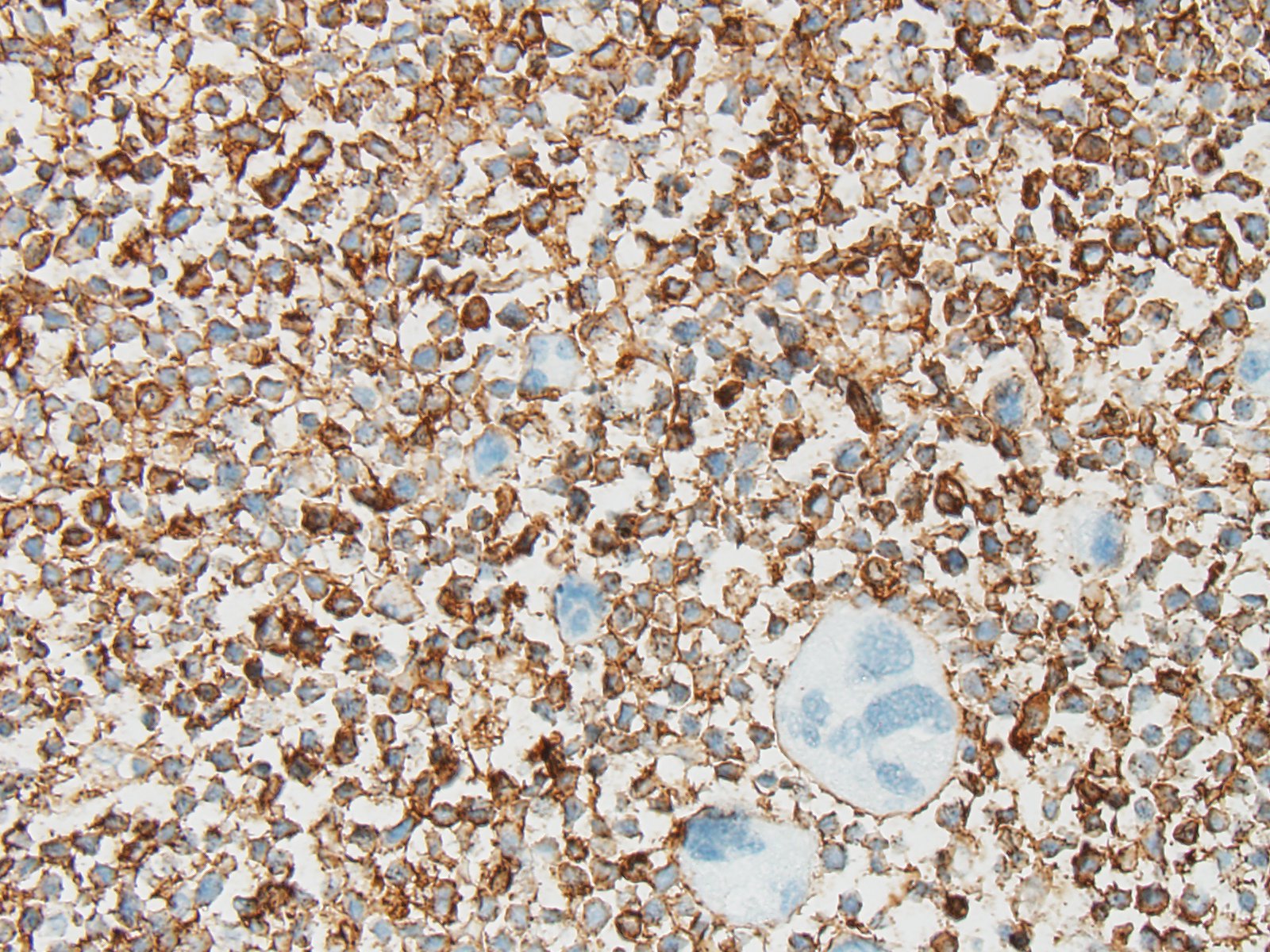
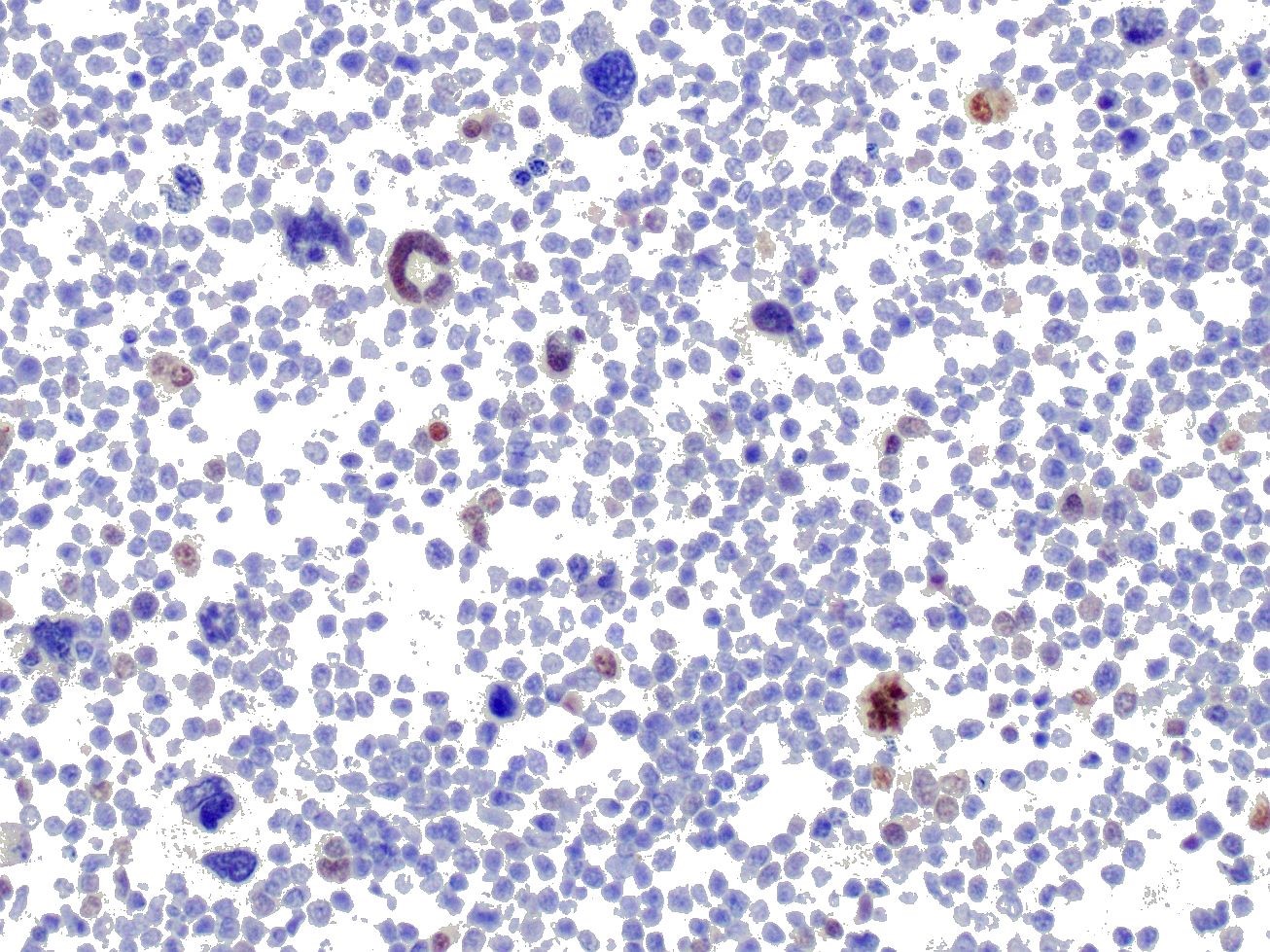
IHC was performed on hepatic sections and confirmed a dual population of neoplastic cells in liver masses. The first population of small round cells had strong cytoplasmic immunopositivity for CD20 (fig. 3), a B lymphocyte marker. Cells surrounding portal areas outside the masses were also CD20 positive. The majority of the second population of larger, pleomorphic cells had moderate cytoplasmic immunopositivity for factor VIII-related antigen (fig. 4), supporting a megakaryocytic origin. Scattered among the neoplastic cells were small numbers of CD3-positive T lymphocytes (not shown).
The CD20 IHC results support a diagnosis of B cell lymphoma, a common disease in dogs. However the presence of the additional population of factor VIII-related antigen-positive cells was unusual. Their presence suggested acute megakaryoblastic leukemia (AML M7; AMegL; AML7), an acute myeloid leukemia in which the predominant blastic cell is of megakaryocytic lineage. Acute megakaryoblastic leukemia (AML M7) is rare but has been reported in humans, dogs and cats. Among canine acute myeloid leukemias it is the least common form, and has previously been described in fewer than 20 dogs. It is a rapidly progressive disease involving bone marrow, internal organs, and lymph nodes. Affected dogs exhibit anorexia, progressive weight loss, and occasionally spontaneous epistaxis. Thrombocytopenia and anemia are frequently observed. Neoplastic cells are typically immunopositive for megakaryocytic markers CD61 and factor VIII-related antigen, and the platelet marker platelet glycoprotein IIIA. They may be variably positive for leukocytic markers CD45 and CD18 and the hematopoietic precursor marker CD34, but are negative for lymphocyte, monocyte and granulocyte markers.
Unfortunately, a potential diagnosis of AML M7 could not be investigated further since neither blood nor bone marrow were available and because financial constraints restricted IHC use. The findings in this case suggest that two forms of hematopoietic neoplasia were present simultaneously and emphasize the need for bone marrow and blood evaluation to achieve a definitive diagnosis.
Contributing Institution:
Diagnostic Services Unit
University of Calgary Veterinary Medicine
Clinical Skills Building
11877 85 St. NW
Calgary AB T3R 1J3
http://vet.ucalgary.ca/
JPC Diagnosis: Malignant plasm cell tumor.
JPC Comment: This is an interesting and unique case. The neoplasm in the liver is composed of CD-20 positive B-cells (the immunostaining was confirmed at the JPC) which are the same size or 1.5x the size of an erythrocyte. Neoplastic lymphocytes have a 3:1 N/C ratio with a thin rim of non-granulated eosinophilic cytoplasm, and mitotic figures are rare (although preservation is not optimal and made it difficult for attendees to reach consensus on grading this neoplasm..) Nucleoli are not evident in well-preserved cells. Immunostains for CD3, PAX-5 and MUM-1 were negative in this particular population of cells.
The large pleomorphic cells scattered throughout the neoplasm exhibited strong nuclear staining for MUM-1 run both at the JPC and at UPenn, suggesting that some of the neoplasm cells may be in a late stage of plasma cell differentiation, and coupled with the CD-20 staining of the smaller uninucleate cells that this neoplasm may represent a late stage B cell or malignant plasma cell tumor. This may also explain the non-specific staining of these cells with Factor VIII (as plasma cells are notorious for non-specific immunostaining). This would also help to explain the absence of these atypical cells outside of the neoplasm (leukemic cells should be in the remainder of the section of liver as well), and the diagnosis of a neoplasm related to myeloma might help to explain the fatal bleeding identified in the clinical history.
The small size of the B-cells, lack of prominent nuclei, and low mitotic rate led some conference participants to a diagnosis of mature peripheral B-cell lymphoma /small lymphocytic lymphoma.) B-cell CLL is primarily a neoplasm of the bone marrow which is characterized by marked lymphocytosis (of small mature lymphocytes). The course of these neoplasms in indolent, and tumors may be found in the spleen, liver, and lymph nodes.
There is also extensive necrosis and loss of hepatocytes throughout the section in centrilobular and some subcapsular) areas. The precise cause of this change is not evident in the examined slide but is strongly suggestive of hypoxia, perhaps due to terminal shock, anemia (perhaps as a result of marrow infiltration by a neoplasm), or resulting from local vascular impairment as a result of the presence of a neoplasm.
References:
- Colbatzky F, Hermanns W: Acute Megakaryoblastic Leukemia in One Cat and Two Dogs. Veterinary Pathology 30: 186-194, 1993
- Comazzi S, Gelain ME, Bonfanti U, Roccabianca P: Acute Megakaryoblastic Leukemia in Dogs: A Report of Three Cases and Review of the Literature. Journal of the American Animal Hospital Association 46: 327-335, 2010
- Park HM, Doster AR, Tashbaeva RE, Lee YM, Lyoo YS, Lee SJ, Kim HJ, Sur JH: Clinical, histopathological and immunohistochemical findings in a case of megakaryoblastic leukemia in a dog. Journal of Veterinary Diagnostic Investigation 18: 287-291, 2006
- Valli VE, Jacobs RM, Parodi AL, Vernau W, Moore PF: Histological Classification of Hematopoietic Tumors of Domestic Animals, 2nd ed. Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology, Washington DC, 2002
- Vernau W, Moore PF: An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Veterinary Immunology and Immunopathology 69: 145-164, 1999