Signalment:
Came back through ER 3/12/09 for pale white gums, murmur, collapse. Current physical exam findings: T=96.1oF, P=150, R=30, MM=pale/white, murmur auscultated, abdomen soft
Gross Description:
- General body condition
- Moderate, diffuse icterus of the subcutaneous fat
- Abdominal cavity
- Moderate diffuse icterus of the abdominal fat
- Mild abdominal effusion
- Moderate, diffuse hepatic fibrosis (presumptive)
- Moderate splenomegaly
- Moderate, diffuse renal icterus
- Thoracic cavity
- Moderate, diffuse lung collapse
- Moderate, focal, right cranial lung lobe emphysema
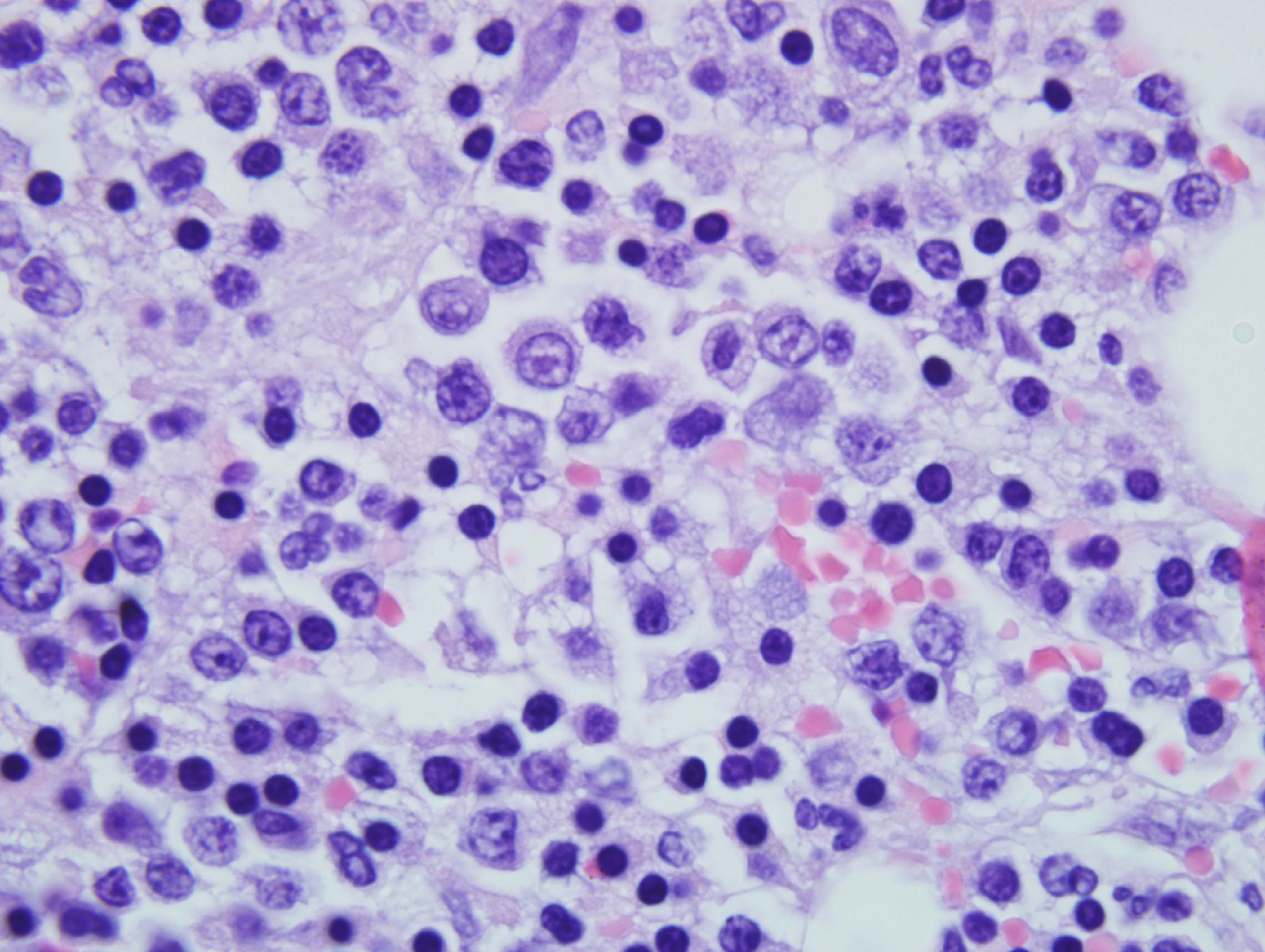
Histopathologic Description:
The neoplastic cells are also observed in the sinusoids of liver, alveolar septae of lungs and in the vascular lumen in brain, kidney, heart and urinary bladder (sections not provided).
Morphologic Diagnosis:
Lab Results:
| TEST | RESULT | REF INT | UNITS | TEST | RESULT | REF INT | UNITS |
| RBC | 2.67 | 5.8 - 10.7 | x106/μl | WBC | 23.2 | 5 - 19.5 | x103/μl |
| PCV | 12 | - | % | Segments | 17.8 | - | x103/μl |
| Bands | 0.6 | - | x103/μl | ||||
| MCV | 47.4 | 39 - 55 | fL | Monos | 2.3 | 0 - 0.85 | x103/μl |
| MCH | 16 | 13 - 17 | pg | Lymp | 2.6 | 0 - 0.75 | x103/μl |
| MCHC | 34.4 | 30 - 36 | g/dl | Atypical nuclear cells | 1.07 | - | x103/μl |
| Platelet | 260 | 175 - 600 | x106/μl | ||||
| Platelet | Clumped > reported value | ||||||
Condition:
Contributor Comment:
EM is characterized by severe non-regenerative anemia with peripheral blood smears revealing variable numbers of metarubricytes, rubricytes, and rubriblasts with markedly decreased mature erythrocytes. In a healthy animal that is not anemic, metarubricytes are a rare occurrence; further, in response to hypoxia or regenerative anemia, increase in metarubricytosis is usually transient. However, lack of significant numbers of circulating polychromatophilic erythrocytes (in Romanowsky- stained blood smears) or reticulocytes (in new methylene blue-stained blood smears) classifies the anemia as nonregenerative. In regenerative anemia, metarubricytosis may be present in acute splenic trauma, post splenectomy, bone fracture or following blood loss.
Pathogenesis of EM has not been completely elucidated, but a positive association is present with infection by Feline leukemia virus (FeLV) type C retrovirus. FeLV is a transmissible retrovirus responsible for or associated with a variety of disease processes. Three subgroups are present, A, B and C. Subgroup A is the infective, horizontally transmissible form of the virus, and subgroups B and C result from viral recombination within individual cats. Subgroup B is primarily responsible for the development of lymphoma and subgroup C is responsible for severe anemia and erythremic myelosis. Though the pathogenesis has not been identified, the prominent finding in EM is a maturation arrest of erythrocytes.(2) The erythrocyte arrest, a key component of EM and other disease, occurs before the reticulocyte stage (figure 1) resulting in severe anemia.
Figure 1.
Rubriblast
↓
Prorubricyte
↓
Basophilic rubricyte
↓
Polychromatophilac rubricyte
↓
Rubricyte
↓
Metarubricyte
----------↓ ----------
Reticulocute
↓
Erythrocyte
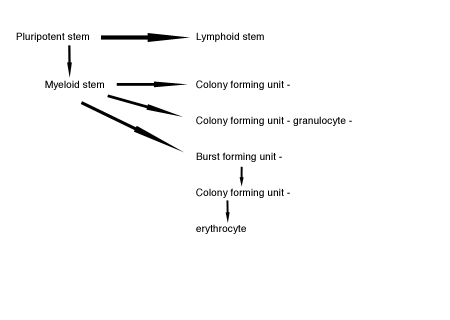
As indicated, above, absence of reticulocytes in the blood of anemic animals is indicative of a disease process at the bone marrow level. A consensus exists that the site of action of the disease in the marrow is at the level of the burstforming and colony-forming units of the erythroid line (Figure 2).(2)
Figure 2.
Clinically, the cats with EM present with depression of days to months, anorexia and mild icterus. The most consistent finding in cats with EM is low hematocrit value of 12 to 15 %. In addition to the severe anemia, the reticulocytes are either in the low-normal range or are not present. As EM is associated with FeLV infection, which is shown to have effects of all three bone marrow cell lines to varying degrees, a complete blood count along with evaluation of peripheral blood smear are indicated.
The numerous circulating nucleated red cells in M6-Er/EM/M6b can superficially mimic the finding in acute hemolytic anemia. In hemolytic anemia, the absence of rubriblasts or very immature erythroid cells in the peripheral blood and an increased reticulocyte count are characteristic of acute hemolysis with regeneration and can aid in differentiation from EM. Also, the marked anemia seen with erythremic myelosis is nonregenerative and rubricytes are in maturation arrest. Feline infectious anemia caused by Mycoplasma haemofelis (previously Haemobartonella felis) is often suspected and may be present but should be recognized as an incidental finding because of the lack of polychromasia and presence of blasts in the blood of cats with EM.(4)
JPC Diagnosis:
Conference Comment:
Most conference participants correctly identified the tissue as bone marrow despite the absence of bone spicules. Conference participants generally agreed there was a proliferation of the erythroid lineage and discussed different classification systems, e.g. the WHO classification system and the French-American-British system.
A differential list for hypercellular bone marrow with erythroblasts and a reduced/absent myeloid component include: 1) myelodysplastic syndromes, 2) myelodysplastic/myeloproliferative diseases, 3) acute myeloid leukemia (e.g. erythremic myelosis, erythroleukemia), and 4) chronic myeloproliferative diseases.(3) Based on the classification system utilized, information from human hematopathology and constant revision to current classification systems, there is some overlap with specific disease entities within each group of disorders.
Myelodysplastic syndromes (MDS) are characterized by maturation defects in clonal stem cells associated with ineffective hematopoiesis and qualitative and quantitative marrow cell dysplasia.(4) These syndromes can evolve into acute myeloid leukemia (AML). Discussion of each syndrome is beyond the scope of this paper and those interested in more information are advised to consult the referenced materials.(3,4) Clinically, animals with MDS present in poor body condition, lethargic, pale mucous membranes and a history of recurring infections, especially involving the respiratory tract. Histologically, the marrow is hypercellular with morphologic change(s) in one or more cell lineages (i.e. erythrocytic, leukocytic and megakaryocytic). Within the marrow, lineages are randomly distributed with loss of clustering of rubricytes. In the cat and the dog, refractory anemia with excess blasts (RAEB) and chronic myelomonocytic leukemia (CMML) are the most common, respectively.(4)
Myelodysplastic/meyloproliferative diseases include entities that may or may not have both myelodysplastic and myeloproliferative features. One such entity, as mentioned above, and showing the malleability of the classification system, is CMML. In the dog, CMML is characterized by leukopenia, nonregenerative anemia, hypercellular marrow and atypical hypersegmented monocytoid cells in blood, bone marrow and lymph nodes. (4) CMML most often occurs in dogs over five years of age. There is often myeloid proliferation with paradoxical neutropenia due to ineffective hematopoiesis. Necropsy findings may include anemia, normal lymph nodes, mild splenomegaly and multifocal hemorrhage due to thrombocytopenia. Differentiation from AML is based on the prolonged clinical course and absence of blast cells in circulation. Two additionally described human diseases which fit into this category, atypical chronic myelogenous leukemia and juvenile myelomonocytic leukemia, are not observed in veterinary species.(4)
Acute myeloid leukemias (AML) are hematologic neoplasms of poorly differentiated cells arising from bone marrow myeloid progenitor cells. Examples of AML resulting in proliferation of cells of the erythroid system include acute erythroid leukemia, M6, which is further separated into erythroleukemia, M6a, and erythremic myelosis, M6b. A diagnosis of AML requires a marrow blast range of greater than 20%; whereas, RAEB requires a marrow blast range greater than 5% but less than 20%. Animals affected with AML are often anemic and thrombocytopenic due to phthis of the marrow by proliferating neoplastic cells and have blast cells present in peripheral blood. There is typically only one proliferating lineage but dysplasia in other cell lines can be observed; prognosis is poorer if dysplastic changes are seen at the time of diagnosis of AML. Additionally, the prognosis is even poorer if animals have been previously diagnosed with myelodysplasia prior to AML diagnosis.(3,4)
Chronic myeloproliferative diseases include chronic myelogenous leukemia, polycythemia vera, essential thrombocytopenia and chronic idiopathic myelofibrosis. Chronic myelogenous leukemia, a disease of aged dogs and cats, is less common than acute myelogenous leukemia. Neutrophils predominate in peripheral blood resulting in a neutrophilia, often with a left shift and nonregenerative anemia.(4)
The moderator provided insight into evaluation of bone marrow specimens. As with histologic evaluation of any organ, understanding of the normal histology is a pre-requisite to identification and description of abnormal. The bone marrow can be divided into the vascular compartment containing arteries, veins and sinusoids and the hemopoietic compartment with the hematopoietic cells and adventitia (adipose tissue and bone).(4) Current human classification identifies six types of MDS while the current WHO fascicle of veterinary hematopoietic tumors identifies three forms. One veterinary clinical pathology text discusses the Animal Leukemia Study Groups three classes of MDS and a modified version by Raskin.(3)
References:
2. Morrison, JA. Erythremic myelosis. Compendium 23:880-886, 2001
3. Stockham SL, Scott MA: Bone marrow and lymph node. In: Fundamentals of Veterinary Clinical Pathology, 2nd ed., pp. 324-358. Blackwell Publishing, Ames, IA, 2008
4. Valli VEO: Hematopoietic system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 125-147. Elsevier Ltd, Philadelphia, PA, 2007