Wednesday Slide Conference, Conference 5, Case 3
Signalment:
7-month old, 141 kg, weaned, female, Brahman calf
History:
The calf was from the Beef Research Unit at the University of Florida (UF) composed of 237 weaned calves ranging from 6 to 8 months of age. The herd had experienced three deaths subsequent to a recent introduction of a post-weaning feed supplement to the animals. The affected calves presented with lethargy, weakness, incoordination, tachycardia, and recumbency. Clinical examination of this particular calf revealed a history of lateral recumbency and weakness within the last 24 hours, moderate dehydration, tachycardia (130 bpm), dyspnea, and ruminal hypomotility (1 weak contraction/min). The animal remained in lateral recumbency and died spontaneously within 24 hours of examination. The calf was submitted to the UF Veterinary Diagnostic Laboratories for necropsy.
Gross Pathology:
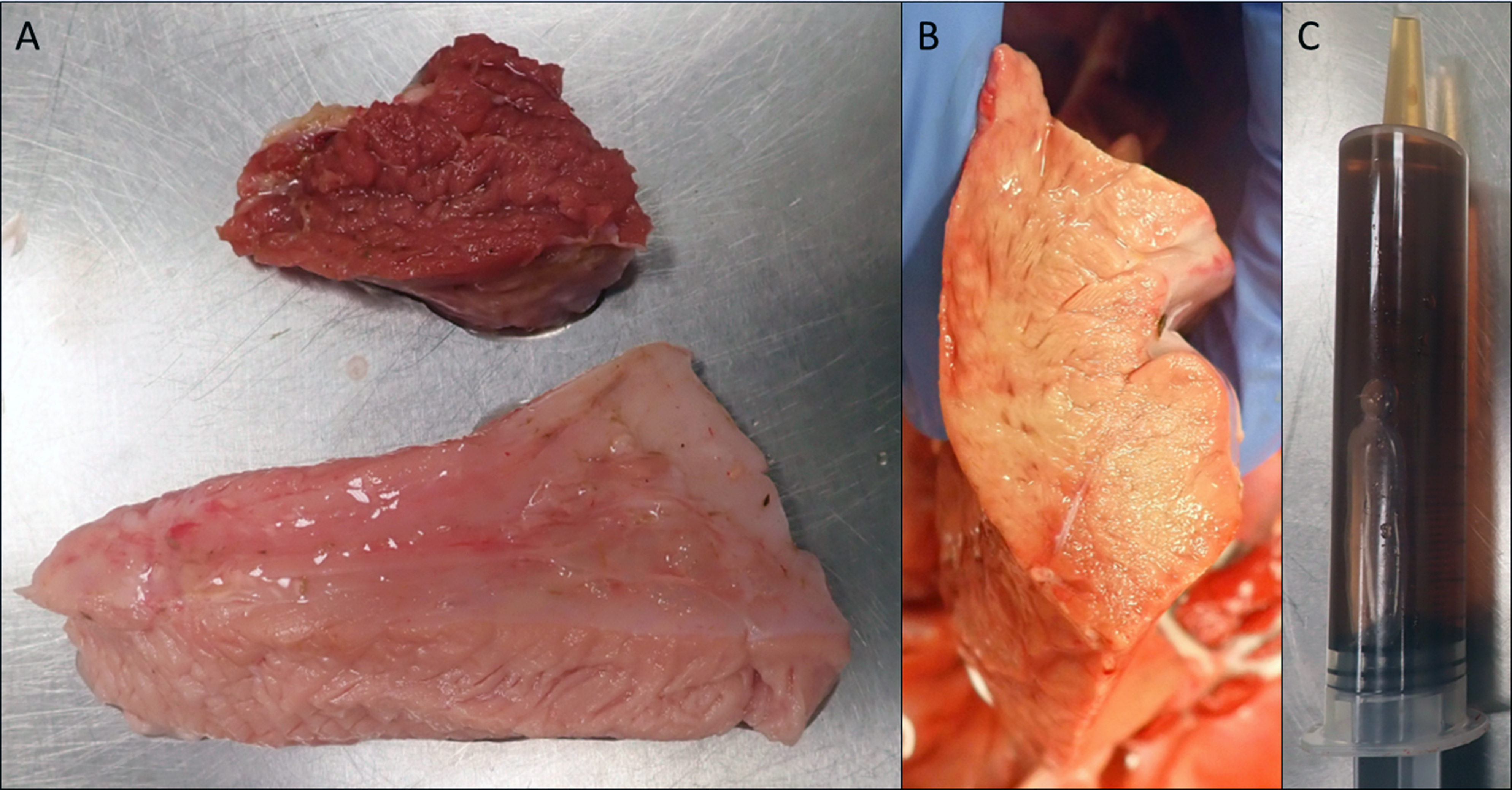
The animal was in good postmortem condition and good nutritional condition, with symmetrical muscling and appropriate subcutaneous and visceral adipose stores. Several muscle groups in the left and right thighs, especially the semimembranosus and semitendinosus muscles, contained multiple, multifocal to coalescing, ill-defined, pale tan streaks. Some skeletal muscle groups exhibited large, pale tan areas of discoloration. The superficial muscle fibers appeared more affected than the deeper ones. The pericardial sac contained mild to moderate amounts of a watery, translucent, yellow-tinged fluid. The epicardial surface of the left ventricle wall had multiple, 1-2 mm, round, flat, dark red foci. The myocardium of the left and right ventricles and interventricular septum contained multiple, multifocal to coalescing, ill-defined, pale tan streaks. The left and right cranial lung lobes had a gelatinous consistency and were mottled dark red to purple. On cut section, those lung lobes oozed a watery, translucent, red-tinged fluid. Samples of all lung lobes floated when placed in formalin. A prominent reticular pattern was observed throughout the liver lobes. The urinary bladder was distended by and filled with moderate amounts of a watery, translucent, brown-tinged fluid. No additional significant gross findings were observed in the remainder of the carcass.
Laboratory Results:
Pertinent laboratory results are indicated below.
Serum chemistry results: Creatine Kinase: 343,240 U/L; Aspartate aminotransferase: 2,335 U/L (ref: 48-139 U/L); Alkaline phosphatase: 181 U/L (ref: 30-69 U/L); Total bilirubin: 0.8 mg/dL (ref: ≤ 0.3 mg/dL); Sodium: 135.9 mEq/L (ref: 140-148 mEq/L); Calcium: 8.6 mg/dL (ref: 9.3-10.8 mEq/L); Chloride: 89.1 mEq/L (ref: 101-113 mEq/L).
Toxicology*: The levels of Monensin detected in two random samples of the feed supplement were 573 ppm and 856 ppm (Recommended label range: 11-33 ppm).
Vitamin E analysis**: The level of vitamin E detected in the submitted liver sample was 61.85 ug/g (Reference range: 7-40 ug/g)
Selenium analysis**: The level of selenium detected in the submitted liver sample was 1.31 ug/g (Reference range: 1.1-5.9 ug/g).
*Toxicology performed at the Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa
**Nutrient analysis performed at the Michigan State University Veterinary Diagnostic Laboratory, Lansing, Michigan
Microscopic Description:
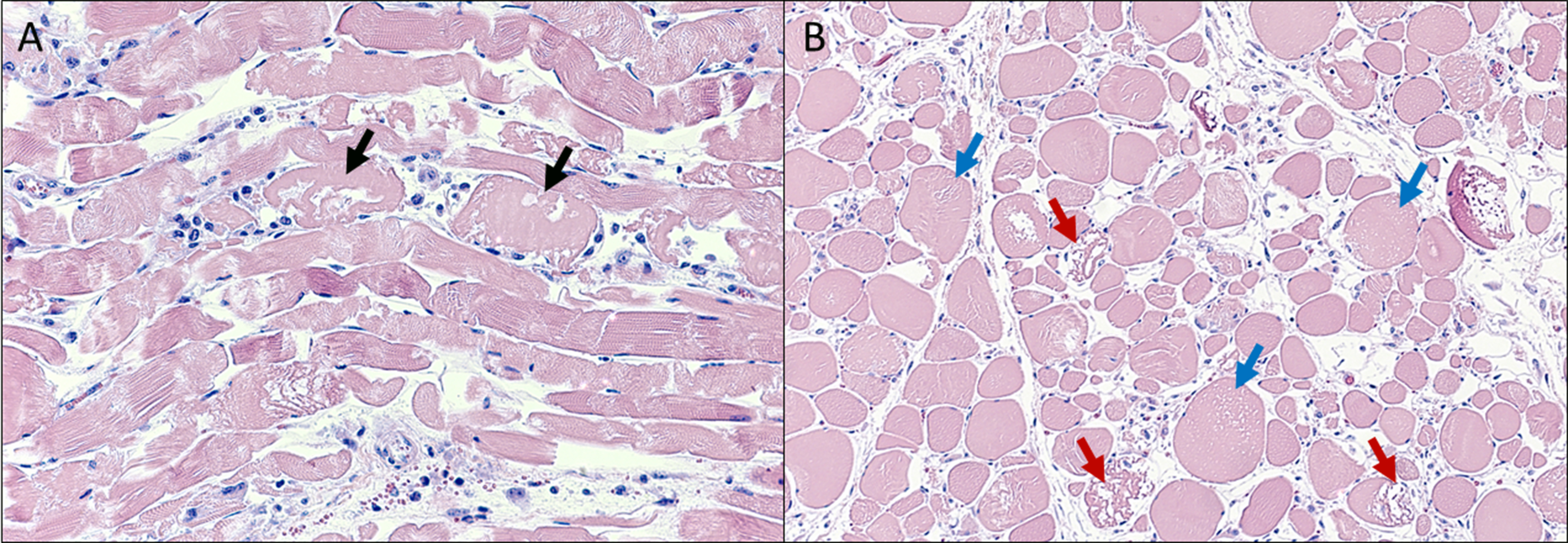
Skeletal muscle: Scattered throughout the musculature, myofibers are mild to moderately enlarged and have a hyalinized, often fragmented, homogeneous, eosinophilic sarcoplasm with loss of myofibrillar striations. In longitudinal sections, the myofibrils are often separated by longitudinal, empty spaces and occasionally lose their regular parallel pattern. In cross-sections, many myofibers contain variably sized, round to ovoid, empty vacuoles within the sarcoplasm. Some myofibers are shrunken, have a highly fragmented sarcoplasm, and are intermixed with pyknotic nuclei and karyorrhectic debris. Throughout the musculature, often between degenerate to necrotic myofibers are low to moderate numbers of macrophages and plump spindled cells (presumptive satellite cells).
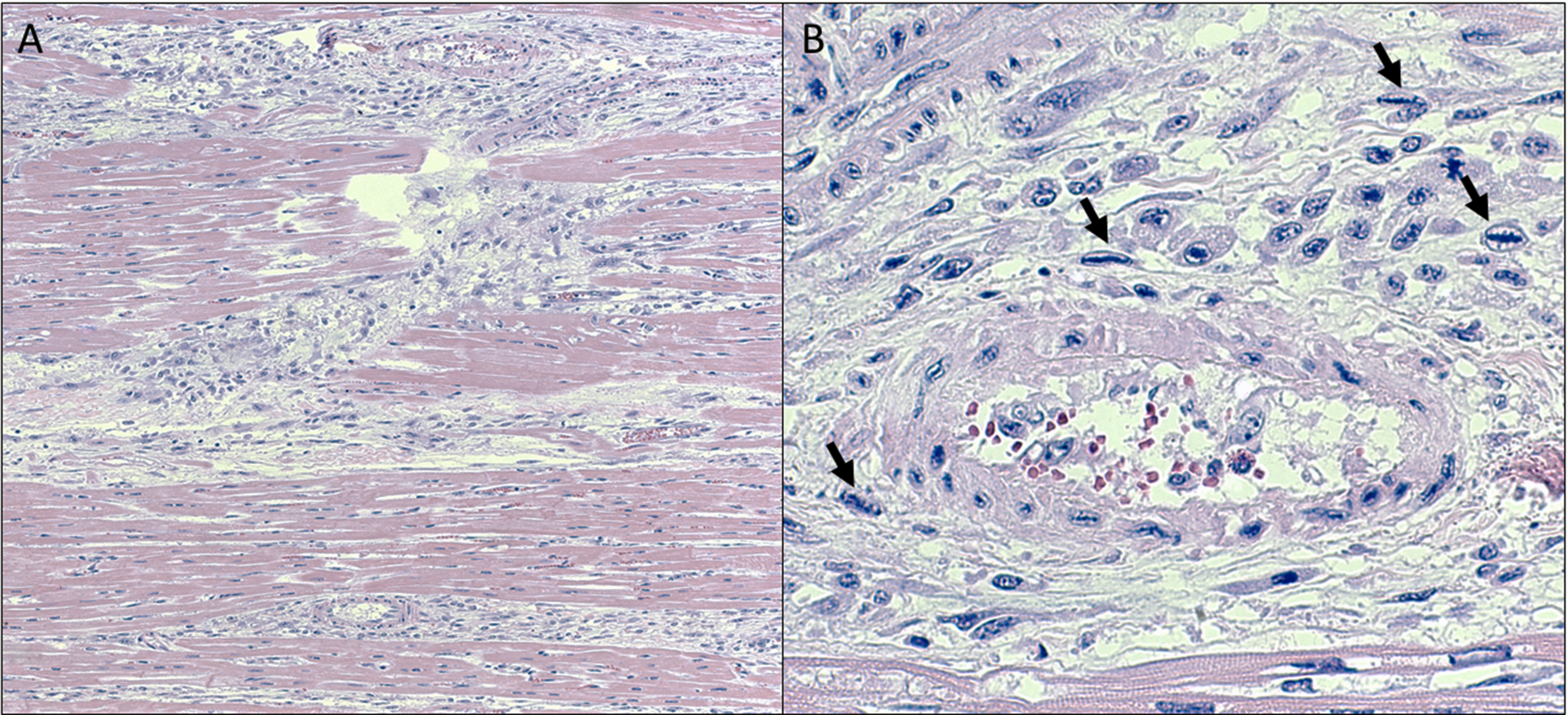
Heart (tissue not included on the slide): The most prominent change was the presence of dense aggregates of plump, spindled cells and macrophages, replacing areas of cardiomyocyte loss. Multiple regions contain individual to small groups of cardiac myofibers with a mildly attenuated to mildly enlarged, mildly hyalinized, homogeneous, bright eosinophilic to amphophilic sarcoplasm with loss of myofibrillar striations. Often the sarcoplasm of affected myofibers is vacuolated. Occasional myofibers are hypereosinophilic with a pyknotic nucleus. Occasionally between myofibers are linear accumulations of an amphophilic granular material admixed with scant to mild amounts of karyorrhectic debris. In the interstitium, often associated with these areas and bordering myocardial vessels, are multiple, often linear, moderate density aggregates of plump spindled to stellate cells, fibroblastic cells, and macrophages. Occasionally these plump spindled cells have a large prominent nucleus with condensed, linear, deep basophilic chromatin (Anichkov cells).
Contributor’s Morphologic Diagnoses:
- Myofiber degeneration and necrosis, acute, multifocal to coalescing, marked, skeletal muscle.
- Myocardial degeneration and necrosis, acute to subacute, multifocal, moderate, heart (tissue not included on the slide).
Contributor’s Comment:
The major histopathologic changes in this calf involved several skeletal muscle groups and the myocardium, explaining clinical evidence of lethargy, weakness, and lateral recumbency noted on physical examination. Moreover, the histologic changes in the skeletal and cardiac musculatures confirm myodegeneration and myonecrosis. The widespread, synchronous muscle necrosis identified is consistent with a multifocal monophasic pattern of necrosis, suggesting a toxic myopathy or metabolic disorder3. Differential diagnoses for myodegeneration and myonecrosis in cattle typically include ionophore toxicosis, ingestion of myotoxic plants (e.g., Senna spp.), and nutritional myopathy.1-3,7,9,13 The high serum levels of creatine phosphokinase, hepatic function dyscrasias, and hyponatremia and hypocalcemia are consistent with muscle necrosis, liver damage, and ionophore-induced influx of sodium and calcium into the cells, respectively. Pulmonary edema, pericardial effusion, and centrilobular necrosis are presumably sequelae to cardiac decompensation. A definitive cause for the cerebral vacuolar change is uncertain but may be related to tissue hypoxia/ischemia associated with cardiac failure.
In this calf, the multifocal monophasic pattern of myonecrosis, history of a newly introduced feed supplement, acute onset of clinical signs, serum chemistry abnormalities, and myocardial involvement would support the diagnosis of ionophore toxicosis.3,4,6,13 Ultimately, this diagnosis was confirmed with the detection of toxic levels of monensin in the feed. The moderately increased levels of vitamin E in the liver and normal hepatic concentration of selenium would reduce the likelihood of nutritional myopathy as the cause of death in these calves. Furthermore, the feed and gastro-intestinal tract lacked plant parts suggestive of Senna spp.
Ionophore is the generic term to describe any lipid-soluble molecule that facilitates the transport of positively-charged ions across biologic membranes.10 Monensin, lasalocid, and salinomycin are typical ionophores of veterinary clinical significance.4,6,11,13. Monensin is produced by the fermentation of Streptomyces cinnamonensis.4,6,13 This ionophore is primarily used in veterinary medicine as an anticoccidial drug in poultry and for growth promotion and production efficiency in cattle.4,6 Its three-dimensional (3-D) structure resembles a doughnut with the cation-binding site at the area of the doughnut hole.10 This 3-D conformation confers some degree of selectivity for sodium and potassium to monensin.10 The mechanism of action of monensin involves several sodium-hydrogen and potassium-hydrogen exchanges to move sodium ions into the cells and potassium ions out of the cells.4,6,10,13
Excessive ingestion of monensin can be toxic to several animal species. However, the susceptibility to monensin toxicosis, estimated by calculating the LD50, varies considerably among species.3,10,14 For instance, the estimated LD50 in horses, cattle, and poultry are 2-3 mg/kg, 50-80 mg/kg, and 90-200 mg/kg, respectively, explaining why horses are very susceptible, and poultry are quite resistant to monensin toxicosis.3,13 The pathogenesis of the disease is directly associated with the mechanism of action of monensin.3,4,6,10,13 Long-standing, monensin-mediated accumulation of sodium into the cells, particularly myocytes and cardiomyocytes, leads to secondary water accumulation within the cytoplasm and organelles, which in turn induces cell swelling and rupture of membranes, ultimately culminating with irreversible cell injury and cell necrosis.3,4,6,10,13 In some instances, the toxic effects of Monensin may be potentiated with certain drugs.1,10 Cattle ingesting nontoxic levels of Monensin in feed contaminated with macrolide antibiotic residue may still develop monensin toxicosis, presumably due to delayed hepatic clearance of monensin, resulting in accumulation and subsequent toxicity.1
Monensin toxicosis often is associated with feed-mixing errors that result in excess levels being added to the feed or access to feed sources destined for another species.4,6,13,14 Grossly, several muscle groups and the myocardium have variably sized, pale tan areas of discoloration, representing areas of multifocal monophasic myonecrosis histologically.3,4 Monensin poisoning may affect animals of all age groups.3,4,6,13 However, heavier and stronger animals are typically the first ones to perish because of the dominant behavior allowing them to ingest more of the contaminated feed. Definitive diagnosis involves the detection of toxic levels of monensin in the suspected diet and sometimes in the ruminal content.4,6,13 The suspected feed is the preferable sample for toxicology as the concentration of monensin in the ruminal content may be lower than that of the feed ingested due to absorption and/or ruminal breakdown4.
Cattle may accidentally ingest Senna spp. when grazing or consuming feed contaminated with the plant.5,8,12,13 Although Senna spp. poisoning typically causes multifocal monophasic necrosis, a similar pattern of necrosis to that induced by ionophore toxicosis,3 absence of this toxic plant in the feedlot pen and beans and/or seeds in the feed and ruminal content would disfavor Senna spp. poisoning. Moreover, cattle grazing forage in soils deficient in selenium and/or being offered feed with low vitamin E content may develop nutritional myopathy.3,7 This condition commonly affects young animals, up to 6 months of age, ingesting poor-quality feed.3 Since the calf, in this case, was in a similar age group and with myodegeneration and myonecrosis, selenium/vitamin E deficiencies were considered possible differentials. However, the typical multifocal polyphasic necrosis encountered with nutritional myopathies was not seen in this case,3 decreasing the likelihood of nutritional myopathy. The moderately elevated levels of vitamin E and normal concentration of selenium in the submitted liver sample excludes selenium and vitamin E deficiencies as the cause of myodegeneration and myonecrosis in this calf.
Interestingly, all affected animals in the herd involved lighter animals, which is not usually the case in diet-related toxic causes where dominant animals may be exposed to a higher toxin dose. However, the animals in this herd were offered feed according to the average daily intake for the group based on the average herd weight. Therefore, by potentially receiving a higher dosage of monensin for their individual weight, it is possible these lighter animals were more susceptible to toxicosis. Deaths were not observed in the herd after discontinuing the monensin-supplemented feed.
Contributing Institution:
University of Florida, College of Veterinary Medicine, Department of Comparative, Diagnostic, and Population Medicine, Gainesville, Florida, USA.
https://cdpm.vetmed.ufl.edu/
JPC Diagnoses:
Skeletal muscle, myofibers: Degeneration and necrosis, monophasic, multifocal, moderate.
JPC Comment:
This case is a good example of a monophasic myonecrosis supported by ancillary diagnostics and a comprehensive writeup from the contributor. The lesions in the submitted slides are significantly less profound than the ones photographed by the contributor, but is consistent with “real-world” lesions in many cases of ionophore toxicosis.
Conference participants offered a number of interesting causes for this case including ionophores, vitamin E / selenium deficiency (white muscle disease), trauma (e.g. downer cow, capture myopathy) and glycogenosis type II. In downer cattle, massive focal necrosis of skeletal muscle is the result of ischemia driven by marked pressure i.e. the weight of the body is sufficient to collapse both venous and arterial blood flow.3 The section examined lacked vascular changes and significant edema that would be anticipated as part of a reperfusion syndrome however. Given that Pompe’s disease (type II glycogenosis; a lysosomal storage disease) has been described in Brahman cattle as a cause of skeletal muscle dysfunction8,11, it does merit consideration in this case. However, the underlying cause is an autosomal recessive gene defect in acid maltase (acid α-glucosidase) with the net effect being the inability to breakdown glycogen within lysosomes during normal cellular metabolism. As such, major effects are noted in glycogen-rich, glucose-dependent tissues such as the heart and CNS with cardiac dysfunction and failure being the ultimate cause of death. The major histologic feature of Pompe’s disease is marked vacuolation of skeletal myocytes, cardiac myocytes, and neurons of the PNS/CNS with vacuolization representing glycogen within lysosomes than can be confirmed via periodic acid-Schiff (PAS) with diastase treatment.8 This is not the predominant microscopic feature in this case.
References:
- Basaraba RJ, Oehme FW, Vorhies MW, Stokka GL. Toxicosis in cattle from concurrent feeding of monensin and dried distiller's grains contaminated with macrolide antibiotics. Journal of Veterinary Diagnostic Investigation, 11(1), 79-86, 1999.
- Blanchard PC, Galey FD, Ross F, Landgraf WW, Meyer H, Spiro N. Lasalocid toxicosis in dairy calves. Journal of Veterinary Diagnostic Investigation, 5(2), 300-302, 1993.
- Cooper BJ, Valentine BA. Muscle and Tendon. In: Maxie, MG. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1, 6th ed. St. Louis, Missouri; Elsevier; 2016:180-182, 208, 212-216, 218-220.
- Ensley S. Ionophore Use and Toxicosis in Cattle. Veterinary Clinics of North America: Food Animal Practice, 36(3), 641-652, 2020.
- Furlan FH, Zanata C, Damasceno ES, et al. Toxic myopathy and acute hepatic necrosis in cattle caused by ingestion of Senna obtusifolia (sicklepod; coffee senna) in Brazil. Toxicon, 92, 24-30, 2014.
- Hall JO, Ionophore use and toxicosis in cattle. Veterinary Clinics of North America: Food Animal Practice, 16(3), 497-509, 2000.
- Kennedy S, Rice DA, Davidson WB. Experimental myopathy in vitamin E- and selenium-depleted calves with and without added dietary polyunsaturated fatty acids as a model for nutritional degenerative myopathy in ruminant cattle. Research in Veterinary Science, 43(3), 384-394, 1987.
- Lyons RE, Johnston DJ, McGowan MR, Laing A, Robinson B, Owen H, Hill BD, Burns BM. E7 (1057ΔTA) mutation of the acidic α-glucosidase gene causes Pompe's disease in Droughtmaster cattle. Aust Vet J. 2017 May;95(5):138-142.
- Nicholson SS. Southeastern plants toxic to ruminants. Veterinary Clinics of North America: Food Animal Practice, 27(2), 447-458, 2011.
- Novilla MN, McClary D, Laudert SC. Ionophores. In: Reproductive and Developmental Toxicology, 2nd Edition. Elsevier; 2017. pp. 503-517.
- Reichmann K, Twist J, Thistlethwaite E. (1993), Clinical, diagnostic and biochemical features of generalised glycogenosis type II in Brahman cattle. Australian Veterinary Journal, 70: 405-408.
- Rissi DR, Barros CSL. Pathology in Practice. Journal of the American Veterinary Medical Association, 250(1), 51-53, 2017.
- Roder JD. Ionophore toxicity and tolerance. Veterinary Clinics of North America: Food Animal Practice, 27(2), 305-314, 2011.
- Silva AWO, Mendonça MFF, Freitas MD, Filho ALR, Silva RDG, Leal PV, Pimentel LA, Peixoto TC. Accidental monensin poisoning in buffaloes in Bahia, Brazil. Pesquisa Veterinaria Brasileira, 42:e06937, 1-9, 2022.