Signalment:
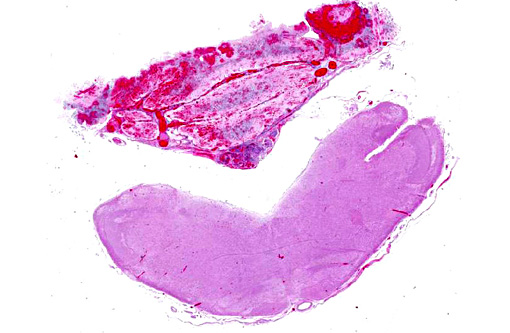
Gross Description:
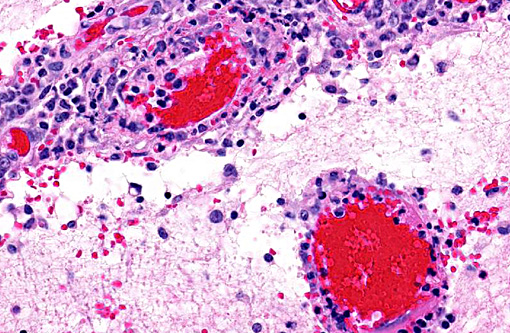
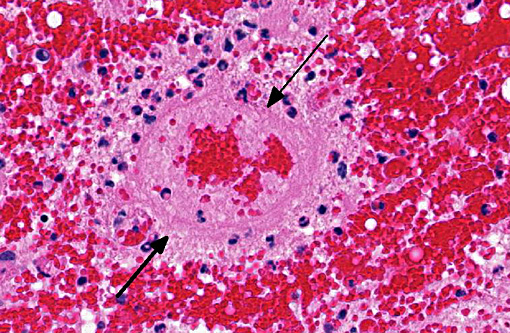
Histopathologic Description:
Morphologic Diagnosis:
1. Cerebellum, choroid plexus, and brain stem: Vasculitis, fibrinonecrotizing and lymphohistiocytic, severe, diffuse, with severe, multifocal hemorrhage, necrosis, edema and thrombosis.Â
2. Lungs (not submitted): Interstitial pneumonia, lymphohistiocytic, diffuse, moderate, subacute.
3. Spleen (not submitted): Splenitis, granulomatous, diffuse, severe with locally extensive necrosis, fibrosis, and multifocal mineralization (chronic infarct).Â
4. Lymph nodes (not submitted): Lymphadenitis, histiocytic, multifocal, moderate, with diffuse lymphoid depletion, and scant intrahistiocytic cytoplasmic botryoid inclusions.
5. Ileum, Peyers patches (not submitted): Lymphoid depletion, diffuse, severe.Â
6. Kidney (not submitted): Nephritis, interstitial, lymphohistiocytic, multifocal, moderate, with tubular degeneration, necrosis and regeneration, and interstitial fibrosis.
7. Tonsil (not submitted): Lymphoid depletion, diffuse, severe.
Lab Results:
Bacteriology: There was no microbial growth from samples of kidney and spleen. A light growth of mixed flora was recovered from the meninges.
Virology: Ileum and tonsils tested positive for porcine circovirus type 2 by FAT and PCR.
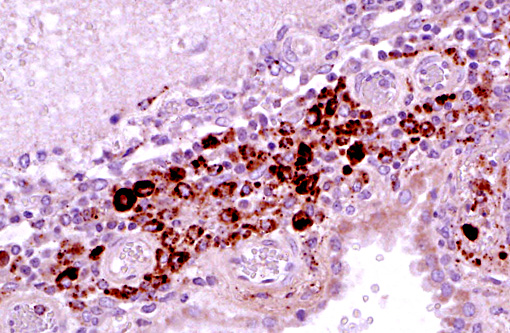
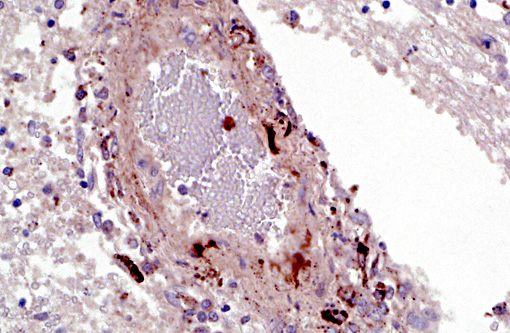
Immunohistochemistry: Porcine circovirus type 2 antigen was present in the inflammatory infiltrate and affected blood vessels of cerebellum, choroid plexus and brain stem (Prairie Diagnostic Services, 52 Campus Dr, Saskatoon, SK. S7N 5B4)
Condition:
Contributor Comment:
Porcine circovirus (PCV) is a non-enveloped, single-stranded, circular DNA virus classified in the Ciroviridae family, genus Circovirus. There are two known PCV species, porcine circovirus type 1 (PCV1) and porcine circovirus type 2 (PCV2). PCV1 is non-cytopathic and non-pathogenic in swine. On the other hand, PCV2 is pathogenic in swine and associated with multiple disease entities. PCV2 is globally distributed and most herds are seropositive for anti-PCV2 antibodies.(8) Porcine circovirus type 2 infection occurs as maternally derived antibodies wane in post-weaned pigs (7-15 weeks of age) and results in either subclinical infection or clinical disease. Clinical disease associated with PCV2 infection can have multiple manifestations which include: post-weaning multisystemic wasting syndrome (PMWS), PCV2-associated respiratory disease, PCV2-associated enteritis, porcine dermatitis and nephropathy syndrome (PDNS), myocarditis/vasculitis, exudative dermatitis,(1,2) and cerebellar vasculitis.(3,11) Subclinical PCV2 infection may occur in apparently healthy pigs but this type of infection can reduce growth performance and increase susceptibility to other pathogens, or decrease the efficacy of vaccines.(9)
The PMWS is a systemic disease that clinically manifests between 7-15 weeks of age. It is characterized by wasting, dyspnea, lymphadenopathy, diarrhea, pallor and jaundice. Coughing, pyrexia, gastric ulceration, and meningitis have also been reported, but are sporadic.(5) Herd morbidity in PMWS herds is variable with 4-30% of the pigs affected. Mortality rates are often high (20-50%). The most consistent necropsy finding in PMWS pigs is generalized lymphadenopathy. PMWS pigs are frequently coinfected with other common bacterial and viral pathogens, and coinfection often complicates gross findings. Microscopically, lymphoid tissues from PMWS-affected pigs exhibit lymphoid depletion and replacement by histiocytic/granulomatous inflammation. Multinucleated (syncytial) giant cells, epithelioid macrophages and macrophage-associated intracytoplasmic botryoid-like basophilic inclusions are also commonly seen in lymphoid tissues (lymph node, tonsil, Peyers patches, and spleen). Focal parenchymal coagulative and apoptotic necrosis in lymphoid tissues has also been described. Microscopic lesions in nonlymphoid tissues are characterized by lymphohistiocytic inflammation and include interstitial pneumonia, hepatitis, interstitial nephritis and enteritis/colitis. The diagnosis of PMWS is based on clinical signs of wasting which may or may not include respiratory distress and icterus, lymphoid depletion and granulomatous inflammation, and the presence of PCV2 antigen or nucleic acid associated with microscopic lesions.(12)
PCV2-associated respiratory disease and PCV2-associated enteritis can often overlap with PMWS or be a contributing factor in porcine respiratory disease complex (PRDC) and bacterial enteritis, respectively. Microscopically, there is lymphohistiocytic interstitial pneumonia sometimes associated with type II pneumocyte hypertrophy and hyperplasia, peribronchiolar fibrosis, and associated lymphoid hyperplasia. Within the intestine, the gut associated lymphoid tissue (GALT; Peyers patches) exhibit similar changes as those described in PMWS. However, the diagnosis of PCV2-associated enteritis is appropriate when there is clinical diarrhea, lymphoid depletion and granulomatous inflammation are present in Peyers patches but not in other lymphoid tissues, and PCV2 DNA or antigen can be demonstrated within the lesions.(2)
Porcine dermatitis and nephropathy syndrome is a distinctive, sporadic, acute clinical entity of growing swine characterized by circular to coalescing, red to purple macules or raised papules and plaques that originate on the skin of the hind legs and perineal region. Lesions may become exudative, crusty, and eventually regress leaving dermal scars. Bilateral swollen kidneys with widely disseminated cortical petechial hemorrhages are also commonly observed. Increased mortality is seen in affected pigs older than three months of age. The typical microscopic lesion is necrotizing vasculitis and glomerulonephritis. Small to medium sized dermal and subcutaneous arterioles are cuffed by neutrophils, macrophages, lymphocytes, and plasma cells that are sometimes present within vascular walls. Arterioles are lined by plump endothelial cells, occasionally occluded by fibrin thrombi and walls can display multifocal hyalinization. Kidney sections exhibit distension of urinary spaces by fibrin intermixed with necrotic cellular debris and hemorrhage, periglomerular and interstitial lymphohistiocytic infiltrates, and distension of renal tubules with cellular and proteinaceous casts. Skin and glomerular lesions are characteristic of a type III hypersensitivity reaction with deposition of immune complexes. Multiple viral and bacterial pathogens have been implicated in PDNS, but PCV2 is considered a contributing.(2)
A striking finding in this particular case was the cerebellar vasculitis, particularly in absence of significant bacterial isolates. It is known that PCV2 produces nonsuppurative or granulomatous encephalitis with gliosis under experimental conditions and the PCV2 antigen has been identified in brain in these cases. However, in cases of naturally occurring encephalitis, PCV2 has been detected in brains of affected animals always in association with other pathogens that can cause encephalitis alone.(7) Cerebellar hemorrhage, edema and necrosis, resulting from necrotizing and lymphohistiocytic vasculitis (and thrombosis) is an uncommon feature that has also been previously described associated with PCV2 infection.(3,11) PVC2 antigen has been demonstrated in the inflammatory infiltrate and affected blood vessels as in this particular case. Similar to this case, the reported PCV2-associated cerebellar vasculitis exhibited some characteristics of PMWS and also changes consistent with PDNS9 suggesting that a type III hypersensitivity reaction with deposition of immune complexes, as it has been considered in PDNS, might be involved in the pathogenesis of this lesion.
JPC Diagnosis:
Conference Comment:
The presence of vasculitis and fibrin in this case led many participants toward an etiology of Streptococcus sues or Hemophilus parasuis. Other differentials to consider for lymphohistiocytic encephalitis and cerebellar hemorrhage include porcine parvovirus, porcine reproductive and respiratory syndrome virus, and hemagglutinating encephalomyelitis virus.(8) Lymphohistiocytic and necrotizing vasculitis is well documented in PCV2-systemic disease, where it occurs most commonly in the mesenteric lymph nodes and kidney though many other organs may also be affected.(10) The virus appears to have a direct cytopathic effect on vascular endothelium, myocytes, and pericytes in these instances, although an indirect mechanism such as a hypersensitivity reaction may also be possible.(10)
References:
1. Chae C: Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet J. 2004;168:41-49.
2. Chae C: A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326-336.Â
3. Correa AM, et al. Brain lesions pigs affected with postweaing multisystemic wasting syndrome. J Vet Diagn Invest. 2007;19:109-12.Â
4. Ellis J. Porcine Circovirus: a historical perspective. Vet Pathol. 2014;51(2):315-327.
5. Harding JC: The clinical expression and emergence of porcine circovirus 2. Vet Microbiol. 2004;98:131-135.
6. Huang YY, Walther I, Martinson SA, Lopez A, Yason C, Godson DL, Clark EG, Simko E: Porcine circovirus 2 inclusion bodies in pulmonary and renal epithelial cells. Vet Pathol. 2008;45:640-644.Â
7. Maxie MG, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kenedy, and Palmers Pathology of Domestic Animals. 5th ed., vol 1. Philadelphia, PA: Saunders Elsevier; 2007:430-431.Â
8. Opriessnig T, Langohr I. Current state of knowledge on porcine Circovirus type 2-associated lesions. Vet Pathol. 2013;50(1):23-38.
9. Opriessnig T, Meng XJ, Halbur PG: Porcine Circovirus Type 2 associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591- 615.
10. Resendes AR, Segales J. Characterization of vascular lesions in pigs affected by porcine Circovirus type 2-systemic disease. Vet Pathol. 2015;52(3):497-504.
11. Seeliger FA, Br+�-+gmann ML, Kr+�-+ger L, Greiser-Wilkie I, Verspohl J, Segales J, Baumg+�-�rtner W: Porcine Circovirus Type 2-Associated Cerebellar Vasculitis in Postweaning Multisystemic Wasting Syndrome (PMWS)-Affected Pigs. Vet Pathol. 2007;44:621634.
12. Sorden SD: Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Hlth Prod. 2000;8:133-136.