Signalment:
Tissues are from a 6-week-old, intact female, Weimaraner dog (
Canis familiaris)This has been going on in my kennel for about 1 year. Puppies will die sometimes with
symptoms of URI and occasionally matted eyes. They have loss of appetite and thirst. For no apparent
reason, it will stop and no puppies will die for several weeks. It then starts again with a lot of deaths.
Adults are now vaccinated every 6 months. Puppies get Bordetella (4 wks), BA2MP (5wks), Parvo (6wks),
DA2PP (7wks). It is not affecting adults, only puppies, usually between 5-7 weeks old.
Gross Description:
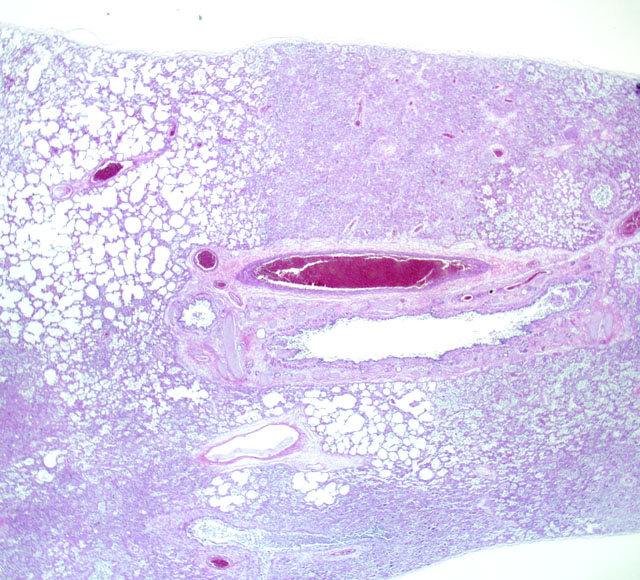
The patient is in relatively good body condition. The eyes are markedly sunken into the
orbits. There is a small amount of vomitus matted in the hair around the nose. Scattered along the ventral
margins of all lung lobes are multiple to locally extensive, 2 mm to 2 cm in diameter dark red foci. These
foci are slightly firm, fail to collapse and extend into the parenchyma on cross-section.
Histopathologic Description:
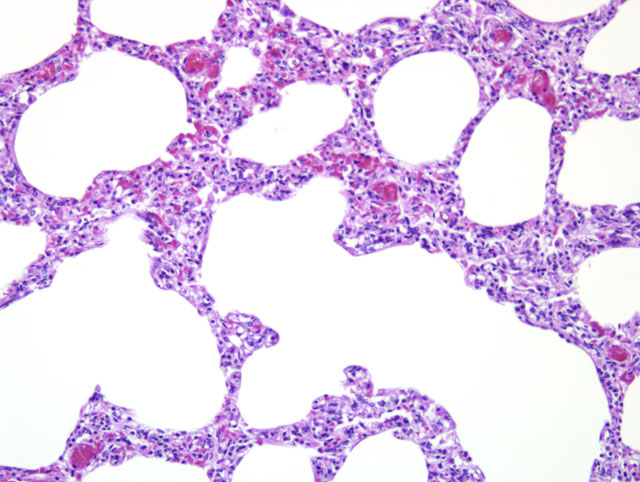
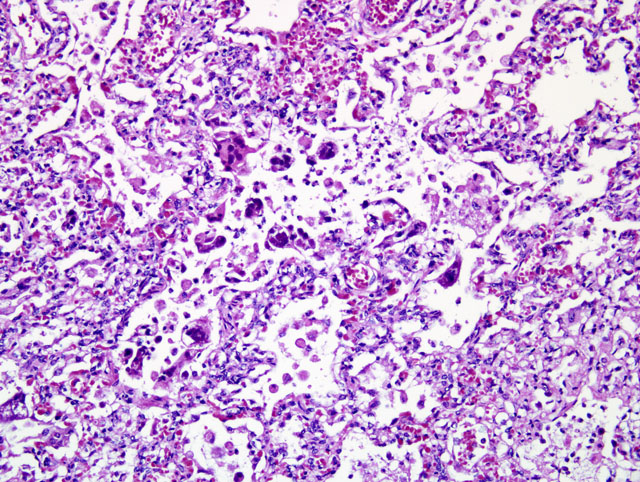
Lung: The normal alveolar architecture is multifocally effaced by areas of
necrosis and inflammation with bacterial cocci, viral syncytia, and viral inclusions. Within affected areas,
there are coalescing aggregates of necrotic cellular debris with numerous foamy macrophages. Frequently,
the macrophages contain large 7-10um diameter basophilic intranuclear inclusions and few 2-5um diameter
eosinophilic intracytoplasmic inclusions. There are frequent syncytial cells that contain up to 5 nuclei.
There are scattered lesser numbers of lymphocytes and neutrophils. The bacterial cocci are 1-2um in
diameter and form small clusters within the area of more intense inflammation. The inflammation
occasionally extends into the lumens of the adjacent bronchioles. The affected bronchioles are often lined by attenuated, ragged epithelium. Within the bronchi, the luminal epithelium is multifocally attenuated. Bronchial epithelial cells occasionally contain 5-7um in diameter oval eosinophilic intranuclear inclusion bodies. Bacterial cocci are occasionally clumped along the lumina surface. Within the less affected areas, the alveoli are flooded with small amounts of fibrin and proteinaceous fluid.
Morphologic Diagnosis:
Lung: Severe, multifocal to coalescing histiocytic necrotizing pneumonia with syncytial cells, intranuclear and intracytoplasmic viral inclusion bodies and bacterial cocci.
Lab Results:
| Bacteriology Results |
| Tissue: Lung | Organism ID: Streptococcus canis
Organism ID: Pseudomonas aeruginosa
|
| Virology Results |
|
Tissue: Lung | | |
| Canine Adenovirus PCR | Positive | Canine adenovirus type 2 |
| Canine Distemper Virus PCR | Positive | Canine distemper virus |
| Fluorescent Antibody Staining |
| Tissue: Lung | Positive | Canine distemper virus |
| Tissue: Intestines | Negative | Canine parvovirus |
| Tissue: Lung | Negative | TGE |
| Tissue: Intestines | Negative | Coronavirus |
| Tissue: Lung | Negative | Herpesvirus |
| Tissue: Lung | Positive | Adenovirus |
Condition:
Canine morbillivirus; Streptococcus canis
Contributor Comment:
The cause of death of this puppy is related to respiratory failure secondary to
the severe pneumonia. There is evidence of concurrent viral and bacterial infections. Most sections exhibit
colonies of bacterial cocci consistent with
Streptococcus canis, which was cultured from lung tissue
collected at necropsy. The presence of intranuclear and intracytoplasmic inclusions in addition to syncytial
formation is diagnostic for canine distemper virus. Morphologically, the character of some of the
intranuclear inclusions was more consistent with adenovirus; additional ancillary testing confirmed a
concurrent adenovirus infection in this puppy.
Individually, canine distemper virus (CDV) is responsible for clinical disease from infection of the
respiratory, gastrointestinal and central nervous systems. In uncomplicated cases, pathogenic strains cause
a bronchointerstitial pneumonia, gastroenteritis that can result in vomiting and diarrhea, and a nonsuppurative
encephalomyelitis with demyelination. The virus has a worldwide distribution and is
particularly prevalent (and generally fatal) in areas where vaccination is not practiced(4). In contrast,
uncomplicated canine adenovirus-2 (CAV-2) infections are seldom fatal. CAV-2 is highly contagious and
in uncomplicated cases results in transient respiratory infections characterized by high morbidity and low
mortality. CAV-2 most important role from a pathogen standpoint is to predispose the patient to bacterial
infection, thus CAV-2 is an important etiological factor in the canine respiratory syndrome of kennel
cough.(4)
Co-infections with CDV and CAV-2 have been reported previously.(2,3,4) In fact, one retrospective study
suggests that co-infections occur more frequently than were previously recognized.(3) The same study also
indicated that histological examination alone is not as reliable for diagnosis of CDV and CAV-2 infections
compared to coupling with ancillary virological testing, primarily because viral inclusions bodies cannot be
demonstrated in all cases.(3)
Interestingly, this puppy as well as the subjects of the previous case reports (2,4) had all been vaccinated
for CDV and CAV. The development of infection and disease in the vaccinated dog may be related to
vaccine failure, reversion of the vaccine strain, immune incompetence to respond to the vaccine, or perhaps
infection occurred prior to vaccination.(2)
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, necrotizing, multifocal to coalescing, severe, with
syncytia, occasional colonies of coccobacilli, and eosinophilic intranuclear and intracytoplasmic inclusion
bodies and large basophilic intranuclear inclusion bodies, etiologies consistent with canine morbillivirus
and canine adenovirus type 2
Conference Comment:
Canine distemper virus, from the genus
Morbillivirus in the family Paramyxoviridae, infects a wide range of species including canids, felids, procyonids, and mustelids, with ferrets being exquisitely sensitive to this virus. Canine Distemper Virus (CDV) is transmitted via
inhalation of infected aerosols, and the virus enters macrophages within the respiratory tract within the first
day of infection. The virus spreads to local lymph nodes and other lymphoid organs within 2-5 days post
infection, and from there the virus uses the bloodstream to gain full access to its host. This stage of
infection is critical in the development of CDV. If a strong cell mediated and humoral immune response is
mounted, the virus is cleared by 14 days post infection with minimal to no viral shedding. If a partial
immune response is mounted, the virus spreads to the respiratory and neurologic systems. Clinical signs
may be minimal, but viral shedding due to infection of the epithelium of the respiratory tract are a sequelae.
There may also be neurologic manifestations in dogs that mount a partial immune response. In dogs that
mount a poor immune response, gastrointestinal, respiratory, and neurologic disease are the result with
copious secretion of virus in feces, urine, and respiratory secretions.(1)
CDV is a unique virus because it is one of the few viruses that cause intranuclear and intracytoplasmic
inclusions. Inclusion bodies within the central nervous system are eosinophilic and intranuclear. In other
infected tissues, inclusions are usually intracytoplasmic. Inclusions are most obvious at 10-14 days post
infection with waning visibility by 5-6 weeks post infection. Inclusions normally can be seen within the
central nervous system after this initial 5-6 week period. Within infected cells of the respiratory tract,
inclusions are most easily seen within bronchial and bronchiolar epithelial cells. Syncytia, if present, are a
key diagnostic feature within affected epithelium. In acute disease, inclusions are often seen within the
urinary bladder and renal pelvis transitional epithelium.(1)
References:
1. Caswell JL, Williams KJ: Respiratory system.Â
In: Jubb, Kennedy and Palmer's Pathology of Domestic
Animals, eds. Maxie ME, 5th ed., pp. 635-638. Elsevier, Philadelphia, PA, 2007
2. Chvala S, Benetka V, Mostl K, Zeugswetter F, Spergser J, Weissenbock H. Simultaneous canine
distemper virus, canine adenovirus-2, and
Mycoplasma cynos infection in a dog with pneumonia. Vet
Pathol. 44: 508-512, 2007
3. Damian M, Morales E, Salas G, Trigo FJ. Immunohistochemical detection for antigens of distemper,
adenovirus and parainfluenza viruses in domestic dogs with pneumonia. J Comp Path. 133: 289-293, 2005
4. Rodriguez-Tovar LE, Ramirez-Romero R, Valdez-Nava Y, Nevarez-Garza AM, Zarate-Ramos JJ,
Lopes A. Combined distemper-adenoviral pneumonia in a dog. Can Vet J. 48:632-634, 2007