Signalment:
Gross Description:
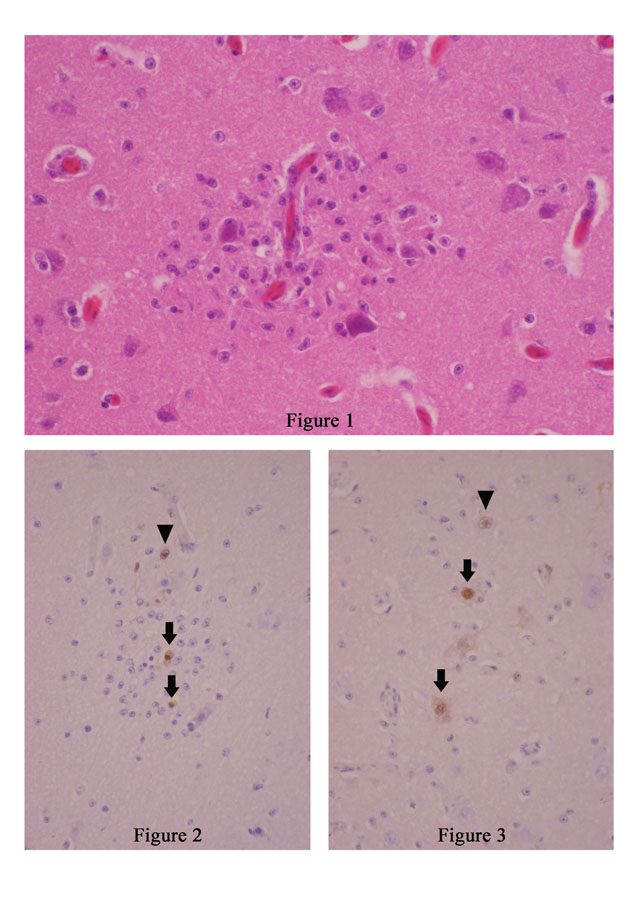
Histopathologic Description:
Imunohistochemistry using rabbit polyclonal antibody against highly pathogenic avian influenza virus of H5N2 subtype as primary antibody revealed viral antigens in the nuclei of astrocytes (arrow head in Fig. 1-2 and 1-3), microglial cells (arrows in Fig. 1-2), and nerve cells (arrows in Fig. 1-3) within and around the glial nodules.Â
Besides the brain, lymphocytic necrosis in the spleen, mild fibrinous bronchopneumonia and focal necrosis of exocrine pancreas were found with viral antigens in alveolar epithelial cells, bronchial epithelial cells and exocrine pancreatic cells. Inflammatory cell infiltration was minimal in these lesions.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Pathological changes of birds due to highly pathogenic avian influenza of H5N1 subtype are necrotic and hemorrhagic changes are centered in the CNS, pancreas, lungs, liver, adrenals, heart and lymphoid organs.(2-4,9,10) The CNS lesions in the present case were very mild and were at an early stage of encephalitis in comparison with previous reports on experimental or non-migratory birds.(2,4)
Birds infected with highly pathogenic avian influenza rapidly develop viremia, and then the virus infects and damages vascular endothelial cells (3,8,9) resulting in hemorrhagic and edematous changes in various organs and tissues including the skin and skeletal muscles. Necrotic and apoptotic changes of parenchymal cells of organs follow. In the CNS, the virus antigen first appears in vascular endothelial cells, then extends to astroglia and nerve cells.(8, 9) In mice, the virus causes neither viremia nor endothelial damage. It invades the CNS via peripheral nerves.(5,7)
JPC Diagnosis:
Conference Comment:
The reservoir for influenza A is waterfowl, and the virus causes an asymptomatic infection in these species with replication in the intestinal epithelium and subsequent fecal shedding. These problematic waterfowl often spread influenza via migratory routes, and the virus has a chance to exchange genes with other novel influenza viruses during these sojourns creating a potential for a new, virulent influenza virus.(6) Swine are important intermediate hosts because they can get both influenza A and C, and thus create an environment for viral genetic rearrangement. Influenza viruses can either undergo genetic drift, (point mutation) or genetic shift (genetic segment reassortment) to create new strains of virus. Most combinations of influenza virus are non-pathogens, but when a drift or shift occurs creating a novel, virulent virus, pandemics such as the 1918 outbreak are the result.(6)
Highly pathogenic avian influenza in chickens and turkeys, also known colloquially as fowl plague, often causes death with little to no clinical warning. If birds survive the initial stage of disease they clinically present with severe respiratory distress along with cyanosis of the unfeathered skin to include the comb and wattles(6) In birds, unlike mammals, influenza replicates in both the respiratory and gastrointestinal tracts. Virulent strains cause viremia with resultant necrosis of lymphoid and gastrointestinal tissue, pancreatitis, myositis, and encephalitis. Petechial hemorrhages are commonly found in the digestive, respiratory and cardiac tissues because of viral damage to endothelial cells.(6)
References:
2. Kobayashi Y, Horimoto T, Kawaoka Y, Alexander DJ, Itakura C: Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol 25:285-304, 1996
3. Kwon YK, Joh SJ, Kim MC, Lee YJ, Choi JG, Wee SH, Sung HW, Kwon JH, Kang MI, Kim JH: Highly pathogenic avian influenza in Magpies (Pica pica sericea) in South Korea. J Wildlife Dis 41:618-623, 2005
4: Le Gall-Recule G, Briand FX, Schmitz A, Guionie O, Massin P, Jestin V: Double introduction of highly pathogenic H5N1 avian influenza virus into France in early 2006. Avian Pathol 37: 15-23, 2008.Â
5. Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, Umemura T: The invasion routes of neurovirulent A/Hong Kong/483/97(H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Vorol 147:1425-1436, 2002
6. Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H, Umemura T: The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet Pathol 41:101-107, 2004
7. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ: Orthomyxoviridae. In: Veterinary Virology, 3rd ed., pp. 459-46. Academic Press, San Diego, California, 1999
8. Park CH, Ozaki H, Takada A, Kida H, Ochiai K, Umemura T: Primary target cells of virulent strains of type A influenza virus in chicken embryos. Avian Pathol 30:269-272, 2001
9. Silvano FD, Yoshikawa M, Shimada A, Otsuki K, Umemura T: Enhanced neuropathogenicity of acian influenza A virus by passages through air sac and brain of chicks. J Vet Med Sci 59:143-148, 1996
10. Teifke JP, Klopfleisch R, Globig A, Starick E, Hoffmann B, Wolf PU, Beer M, Mettenleiter TC, Harder TC: Pathology of natural infections by H5N1 highly patyogenic avian influenza virus in Mute (Cygnus olor) and Whooper (Cygnus Cygnus) swans. Vet Pathol 44:137-143, 2007