Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 6
October 4th, 2017
CASE II: 12060787 (JPC 4033563).
Signalment: 26-day-old, male and female, commercial white broiler chickens, Gallus gallus domesticus, avian.
History: Five birds were submitted from a commercial broiler facility for evaluation for gangrenous dermatitis. Three birds were deceased and two were alive at time of submission. No other history available.
Gross Pathology: Per submittal request, there were no lesions consistent with gangrenous dermatitis in the birds. Instead, the livers were swollen, friable, pale and riddled with dark red to light yellow military foci. Additionally, the kidneys were pale, swollen and friable and the spleen was mottled.
Laboratory results: Acid fast stains on tissues were negative.
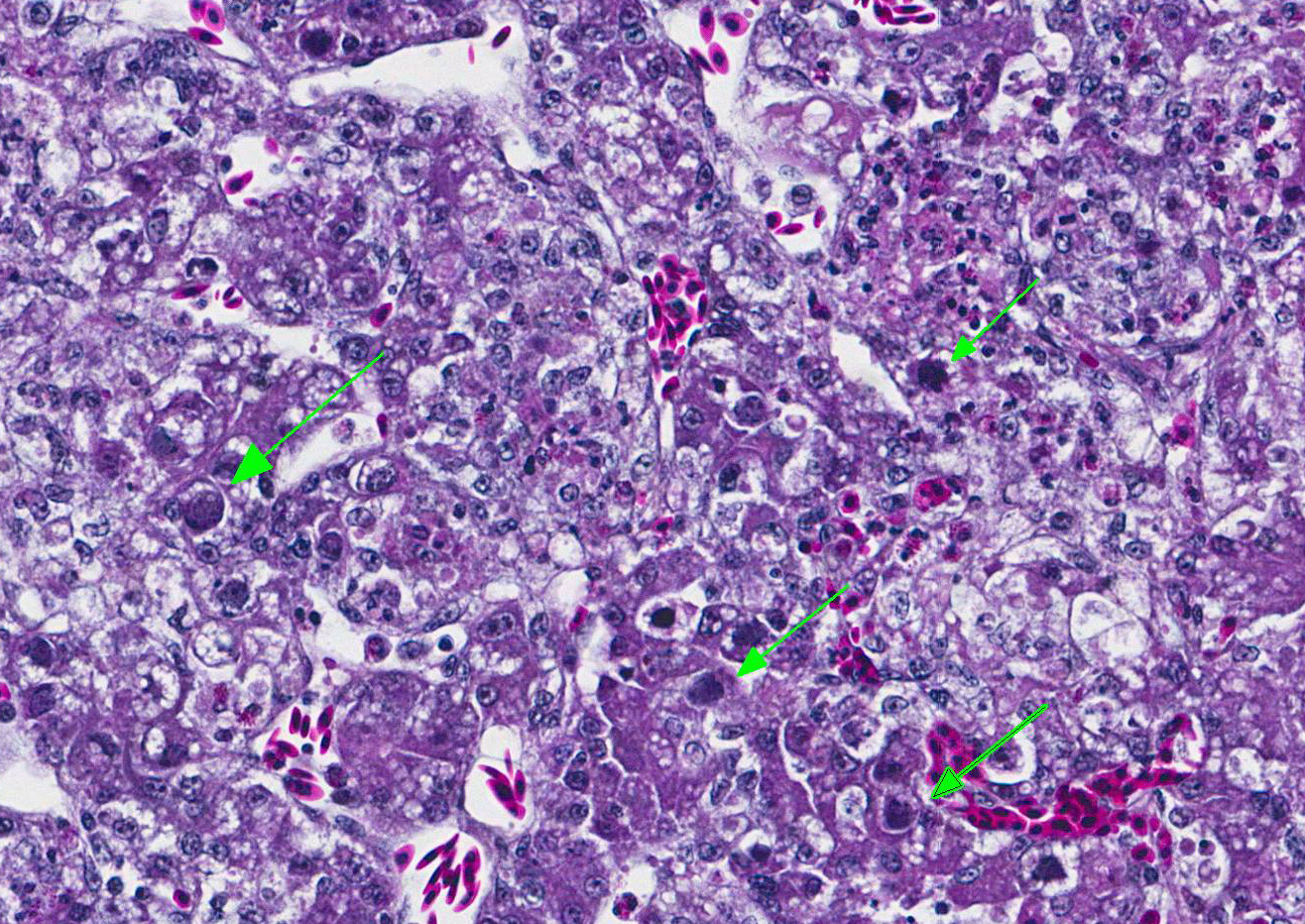
Microscopic Description: Liver: The hepatocellular parenchyma is interrupted by numerous, individual and coalescing foci of necrosis. The foci of necrosis are abruptly demarcated from surrounding viable parenchyma and contain necrotic cell debris along with infiltrates of intact and degenerate heterophils and scant erythrocytes (hemorrhage). Typically restricted to the margin of the viable:necrotic parenchyma, several hepatocytes exhibit large, granular to homogeneous, basophilic intranuclear inclusion bodies that peripherally displace chromatin. Lastly, multifocal portal regions have mild to moderate infiltrates by lymphocytes, plasma cells and fewer macrophages.
Contributors Morphologic Diagnosis:
Liver: Marked, acute to subacute, necrotizing hepatitis with basophilic, intranuclear, inclusion bodies consistent with adenovirus (inclusion body hepatitis).
Contributors Comment: Inclusion body hepatitis (IBH) was first reported from an outbreak in chickens in the United States by Helmboldt and Frazier.3 Since that time, IBH has grown to become a ubiquitous disease with worldwide distribution. IBH occurs in chickens 3-7 weeks of age and is characterized by an abrupt increase in mortality that lasts 3-5 days and can approach 10-30%.2,5 Affected birds exhibit an enlarged mottled liver, icterus, hemorrhages, and pale, swollen kidneys. Microscopic evaluation shows hepatocellular necrosis with eosinophilic or basophilic intranuclear inclusion bodies.
Inclusion body hepatitis is caused by fowl adenovirus. Fowl adenoviruses are divided into at least 12 serotypes, most of which are apathogenic. IBH is typically associated with serotypes 2 and 8; whereas a different clinicopathological manifestation of fowl adenovirus infection, known as hydropericardium syndrome (HPS), is typically associated with serotype 4.2,5 However, some birds have demonstrated both IBH and HPS from administration of the same strain4,and therefore, the clinical disease form may be multifactorial related to background, co-infections, route of infection or age of the bird.
Historically, outbreaks of IBH have been associated with concurrent disease, particularly immunosuppressive agents such as infectious bursal disease (IBD) or chicken anemia virus (CAV).1,6 In the present case, cloacal bursas from affected birds were examined at necropsy and microscopically; no lesions were identified. Additionally, serologic surveys on affected flocks did not demonstrate IBD infection in birds dying from IBH.
A PubMed search for information related to IBH returns a paucity of contemporary studies. Therefore, although this disease may no longer be commonly seen or studied, similar to infectious canine hepatitis (canine adenovirus-1), IBH does re-emerge on occasion.
JPC Diagnosis: Liver: Hepatitis, necrotizing, multifocal to coalescing, marked with numerous basophilic intranuclear viral inclusions, Gallus gallus domesticus, avian.
Conference Comment: There are three genera that affect birds: (1) Aviadenoviruses (group I) which contain fowl adenoviruses (IBH), goose adenoviruses, falcon adenovirus 1, duck adenovirus 2, pigeon adenovirus 1 and turkey adenovirus 1 and 2; (2) Siadenoviruses (group II) which contain turkey adenovirus 3 (hemorrhagic enteritis, marble spleen disease) and raptor adenovirus 1; and (3) Atadenoviruses (group III) which contains duck adenovirus 1 (egg drop syndrome).6 These ubiquitous viruses generally only cause disease in immunosuppressed birds.
Fowl adenovirus, the cause of inclusion body hepatitis, has worldwide distribution, typically affecting young chickens. Most animal species have their own adenovirus that induces hepatitis, enteritis, or respiratory disease (see chart below). With IBH, gross lesions are usually non-specific and may consist of pallor of the wattles and comb, depression, and a sudden increase in mortality within the flock. Depending on the severity of the hepatitis, there may be petechial and ecchymotic hemorrhages in the skeletal muscles of the legs. The liver is generally enlarged with mottling characterized by soft, yellow, focal areas and hemorrhage. Kidneys are often swollen and mottled as well and the Bursa of Fabricius is usually reduced in size. Microscopically, there is focally extensive degeneration and necrosis of hepatocytes with the characteristic basophilic intranuclear viral inclusion bodies within hepatocytes adjacent to areas of necrosis. Renal lesions consist of membranoproliferative glomerulonephritis, and the Bursa of Fabricius is grossly small due to lymphoid depletion.6
Hemorrhagic enteritis of turkeys (caused by turkey adenovirus) results in large basophilic intranuclear viral inclusion bodies within cells of the mononuclear phagocyte system in the spleen leading to widespread necrosis and involution of the white pulp. There is lymphoid depletion in the thymus and Bursa of Fabricius. Intestinal lesions are most prominent in the duodenum and are characterized by mucosal congestion, degeneration and necrosis of the epithelium lining the villus tips and luminal hemorrhage with mixed inflammation in the lamina propria. Adenoviral inclusions are rarely seen in intestinal epithelia, liver, bone marrow, circulating leukocytes, lung, pancreas, brain, and kidney.6
Egg drop syndrome is caused by a hemagglutinating adenovirus (duck adenovirus 1) and results in loss of color of pigmented eggs, decrease in production, or production of thin-shelled, wrinkly eggs in healthy looking laying hens. The virus is widespread in its natural host, waterfowl, and spread vertically and horizontally to domestic birds. In chicks infected in utero the virus remains latent until they start laying eggs. The primary site of replication is in the pouch shell gland and gross lesions other than atrophied ovaries and oviducts are not appreciated. For diagnosis, allantoic fluid can be checked for hemagglutinating activity or viral DNA detected by PCR.6
Table 1: Select adenoviruses in veterinary species4
|
Species |
Name/Species |
Comment |
|
Dogs |
Canine adenovirus
1 |
- Infectious
canine hepatitis |
|
Horses |
Equine adenovirus
1 & 2 |
- Asymptomatic or
mild respiratory disease in immunocompetent hosts |
|
Cattle |
Bovine adenovirus |
- 10 serotypes |
|
Swine |
Porcine
adenovirus |
- 4 serotypes |
|
Sheep |
Ovine adenovirus |
- 7 serotypes |
|
Goats |
Caprine
adenovirus |
- 2 serotypes |
|
Deer |
Cervine
adenovirus |
- Vasculitis, hemorrhage, pulmonary edema |
|
Rabbits |
Adenovirus 1 |
- Diarrhea |
|
Mice |
Murine adenovirus
1 & 2 |
- Murine
adenovirus 1: experimental infections |
|
Guinea pigs |
Guinea pig
adenovirus |
- Usually asymptomatic; rarely pneumonia with high mortality, low morbidity |
Conference attendees also discussed two viral agents that cause immunosuppression and are common concomitant infections in IBH cases: chicken infectious anemia and infectious bursal disease (IBD).
Chicken infectious anemia (Gyrovirus genus, Circoviridae family) is a disease of young birds that is characterized by aplastic anemia, generalized lymphoid atrophy, intramuscular hemorrhage, and immunosuppression. Older birds are usually not affected unless the animal has concurrent infection with infectious bursal disease. The most common method of transmission is vertical from infected hens. Also transmission in feces is common with crowded bird houses. The most common gross lesions are thymic atrophy and yellow, fatty bone marrow. Microscopically, there is thymic lymphoid depletion and atrophy of all cell lines within the bone marrow. Due to resulting immunosuppression, secondary bacterial infections like gangrenous dermatitis may be present.6
Infectious bursal disease (also known as Gumboro disease) is caused by avian birnavirus and results in inflammation and atrophy of the Bursa of Fabricius with resulting immunosuppression. Avian birnavirus has two serotypes with serotype 1 being pathogenic. The virus spreads rapidly, is persistent in the environment, and infects chickens as long as they have a functional Bursa of Fabricius (1-16 weeks old). Initial clinical signs are vague: dehydration, diarrhea, tremor, ataxia, depression, anorexia, and a droopy appearance are common and may resemble coccidiosis. Microscopically, there is marked lymphoid necrosis within the Bursa of Fabricius, thymus, Harderian gland, cecal tonsils, and Peyers patches followed by atrophy and replacement with fibrous connective tissue.6
It was originally thought that IBH was always be preceded by an immunosuppressive pathogen but recently IBH has been accepted as a primary disease.6
Oklahoma Animal Disease Diagnostic Laboratory and the Department of Veterinary Pathobiology
Center for Veterinary Health Sciences
Oklahoma State University
Rm 250 McElroy Hall
Stillwater, OK 74078
References:
1. Fadly AM, Winterfield RW, Olander HJ. Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease viruses. Avian Dis. 1976; 20:467-477.
2. Gomis S, Goodhope R, Ojkic D, Willson P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006; 50:550-555.
3. Helmbolt CF, Frazier MN. Avian hepatic inclusion bodies of unknown significance. Avian Dis. 1963; 7:446-450.
4. MacLachlan NJ, Dubovi EJ. Fenners Veterinary Virology. 5th ed. London, UK: Academic Press; 2017:217-227.
5. Mazaheri A, Prusas C, Voss M, Hess H. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathol. 1998; 27:269-276.
6. Ojkic D, Brash ML, Jackwood MW, Shivaprasad HL. Viral diseases. In: Boulianne M, ed. Avian Disease Manual. 7th ed. Jacksonville, FL: American Association of Avian Pathologists, Inc.; 2013:10-15, 39, 55-56.
7. Philippe C, Grgic H, Nagy E. Inclusion body hepatitis in young broiler breeders associated with a serotype 2 adenovirus in Ontario, Canada. J Appl Poult Res. 2005; 14:588-593.
8. Toro H, Gonzalez O, Escobar C, Cerda L, Morales MA, Gonzalez C. Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis. 2001; 45:215-222.