Wednesdy Slide Conference 2023-2024, Conference 3 Case 2
Signalment:
6-year-old, male neutered domestic short hair, cat (Felis catus)
History:
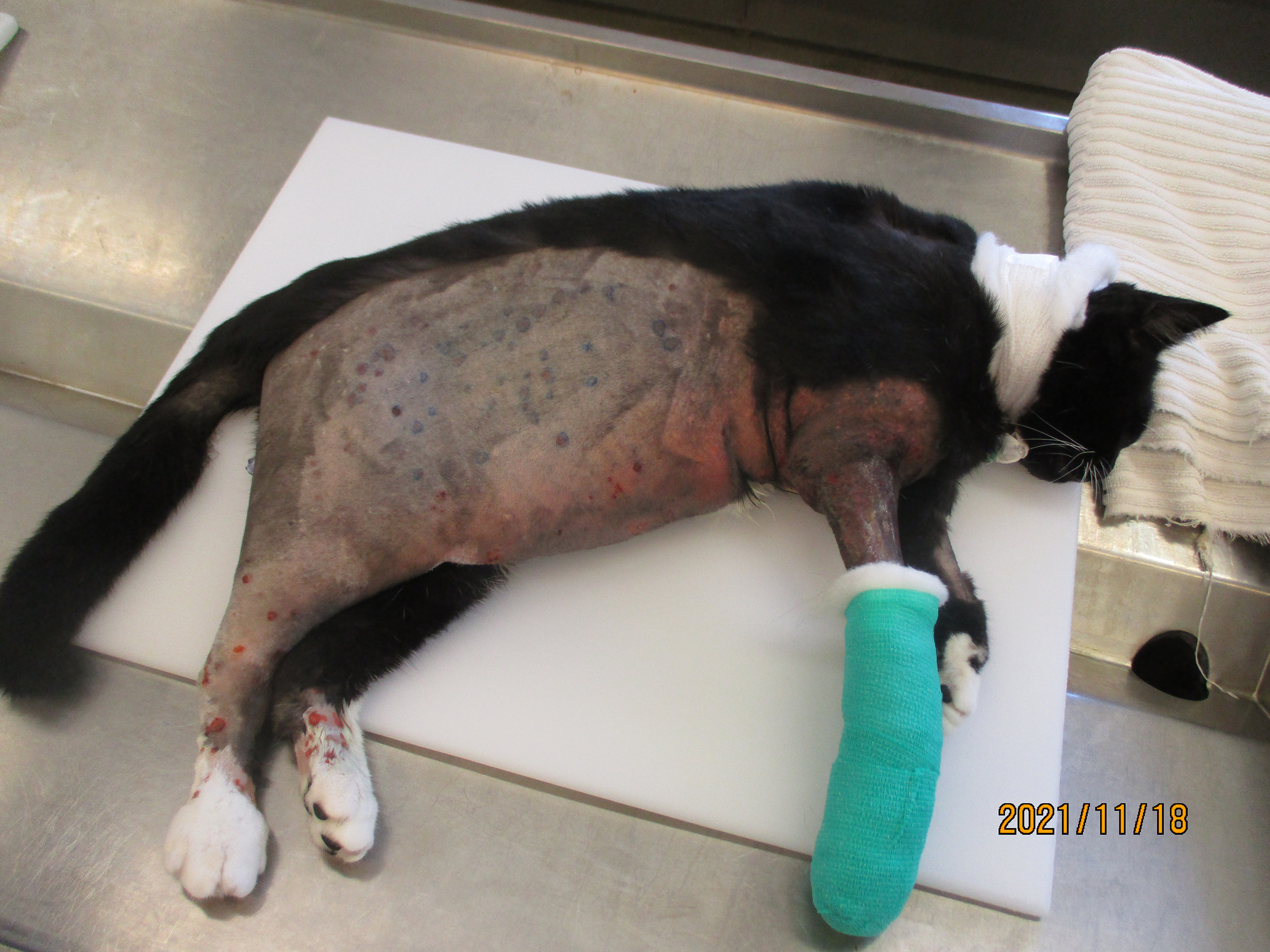
Presented to referring vets for acute onset swelling and lameness of right forelimb with subsequent development of generalised subcutaneous oedema on the right side of the body including the right hindlimb. The cat has outdoor access and no travel history (from the UK). The case was referred for medical investigation following further deterioration. Initial haematology and biochemistry revealed marked leukocytosis, neutrophila and basophilia, and a marked hypoalbuminaemia. Platelet count was normal but with a prolonged PT and aPTT and elevated D-dimers. Full body CT scan demonstrated marked multifocal subcutaneous and intramuscular oedema. Hepatic, splenic and peripheral lymph node cytology demonstrated neutrophilic inflammation and reactive lymphoid tissue. Culture of oedema fluid revealed a moderate growth of alpha-haemolytic colonies resistant to amoxicillin/clavulanate, cephalexin, penicillin G, TMPS and erythromycin, and sensitive to doxycycline, gentamicin and oxytetracycline. Further clinical destabilisation and an uncertain prognosis prompted elective euthanasia.
Gross Pathology:
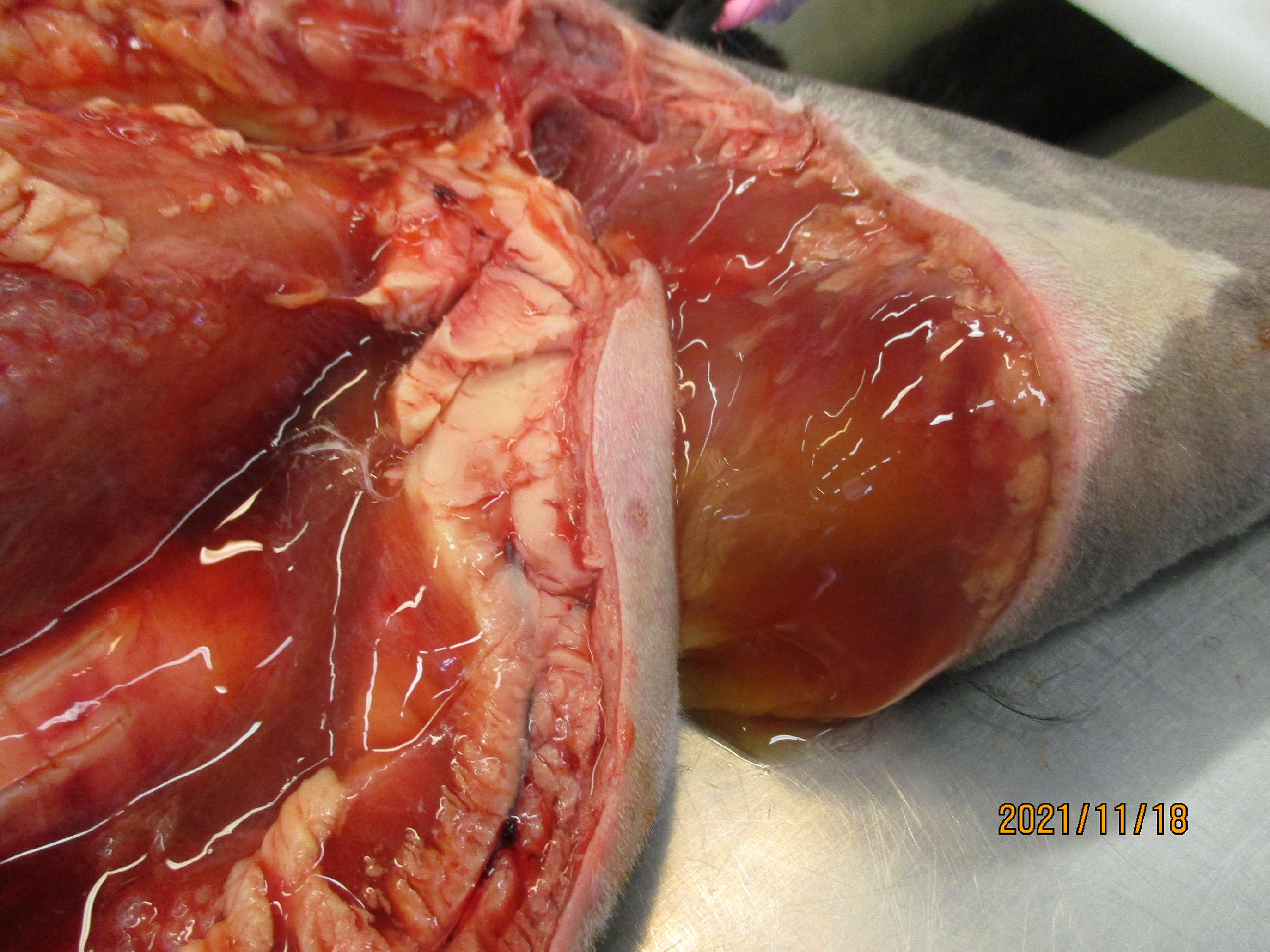
Extensive subcutaneous oedema, multifocal small dermal papules, erosions and ulcers, areas of fascial haemorrhage overlying antebrachial muscles, thoracic effusion and right prescapular lymphadenomegaly.
Laboratory Results:
Feline poxvirus qPCR: detected, 19.08 Ct units. (the higher the Ct, the lower amount of DNA present in the sample; Ct values typically range from 15 to 40; 40 indicates much lower DNA levels than 15).
Microscopic Description:
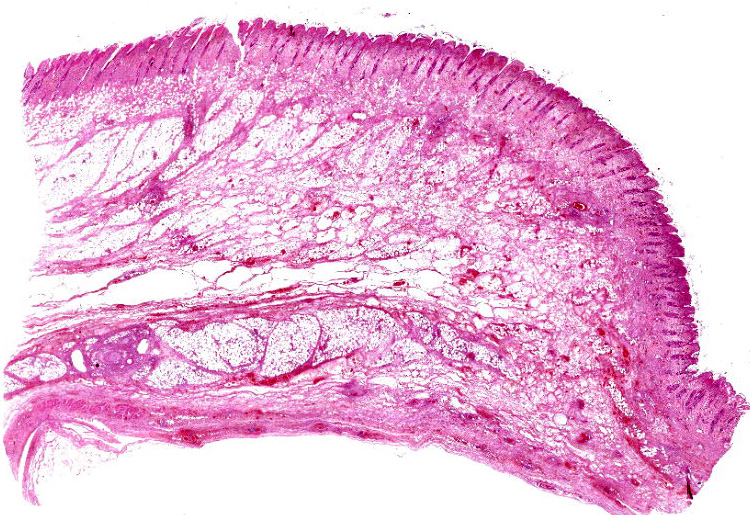
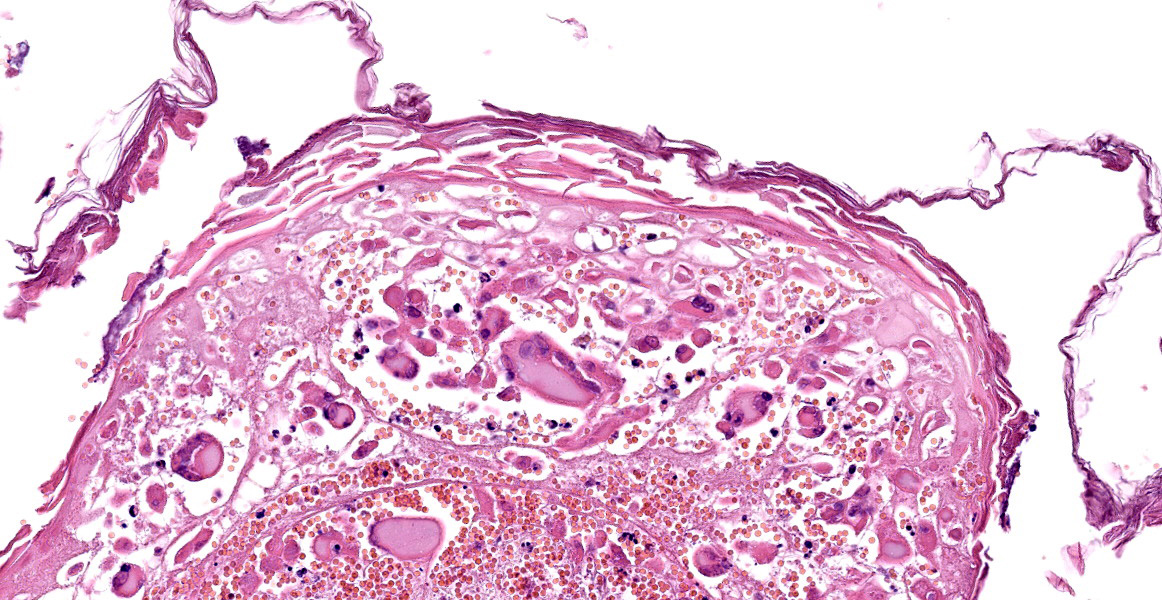
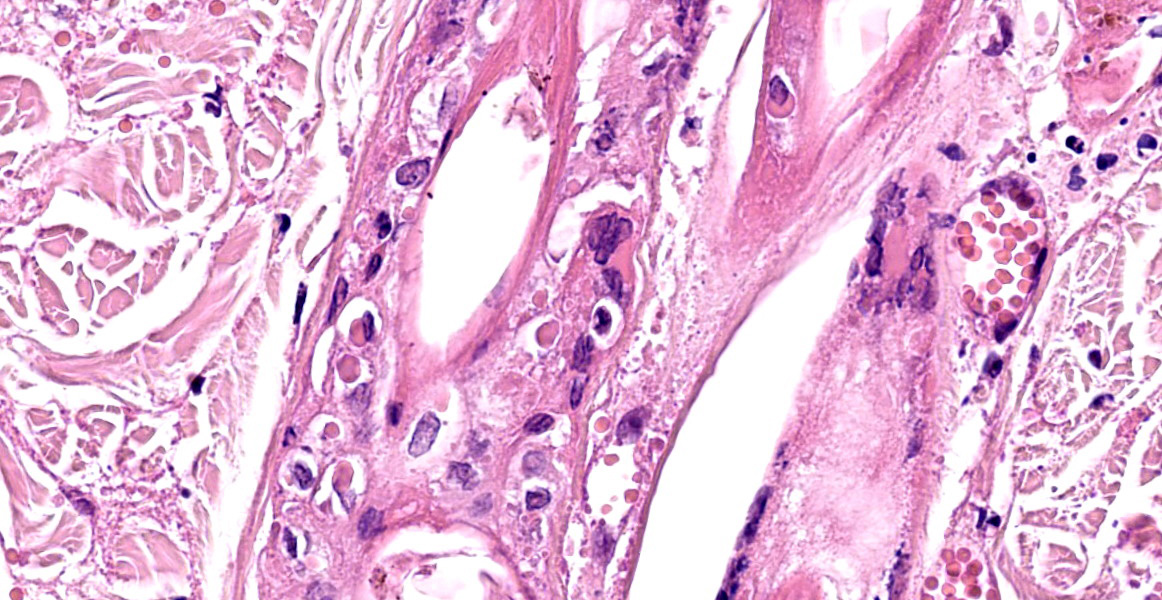
Haired skin and subcutaneous adipose tissue: Affecting 100% of the tissue, the epidermis has lost normal structure. Multifocally there is extensive epidermal necrosis, characterised by keratinocytes with karyorrhectic and karyolytic nuclei, loss of cellular detail, and replacement by eosinophilic debris (necrotic debris and fibrin), nuclear debris and haemorrhage. In areas with viable keratinocytes, keratinocytes demonstrate hypereosinophilia and ballooning (ballooning degeneration), with cytoplasm often expanded by eosinophilic inclusion bodies (type A inclusion bodies). Keratinocytes are also disassociated, with keratinocyte necrosis and loss resulting in reticular degeneration. There are frequent viral syncytia, and these often have nuclei peripheralized by intracytoplasmic eosinophilic inclusion bodies. The epidermis and dermis are expanded by haemorrhage, fibrin and wispy eosinophilic material (oedema).
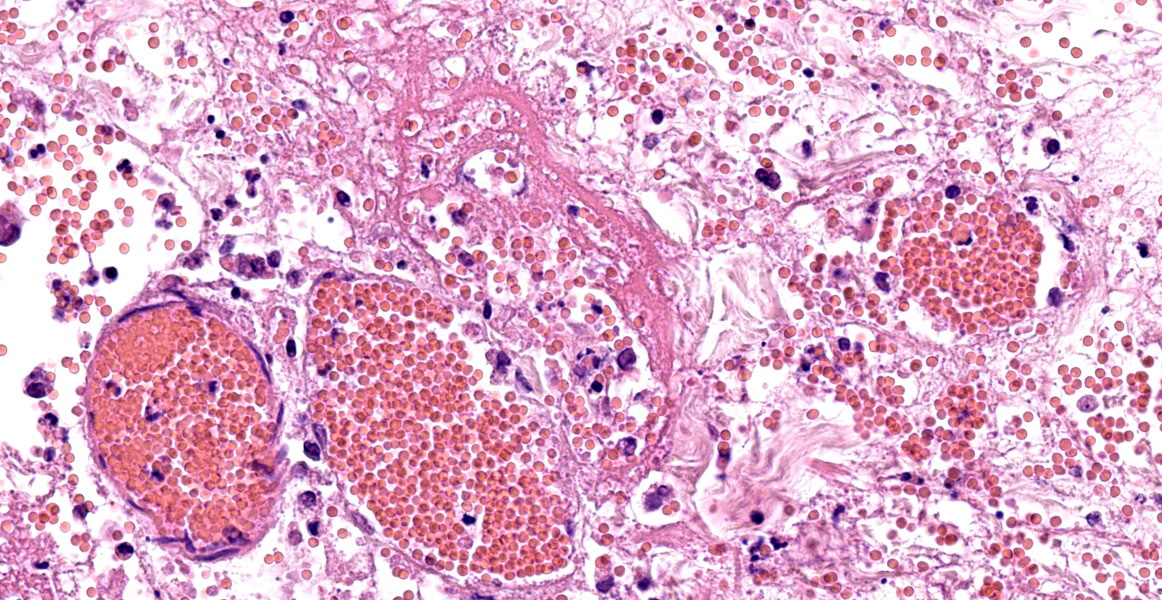
The same epidermal changes, including the necrosis, extend into hair follicles, affecting all levels of the follicular epithelium. At the lower levels of the hair follicles, where there are more viable cells, follicular epithelial cells demonstrate cytoplasmic ballooning that is often accompanied by eosinophilic intracytoplasmic inclusion bodies, and viral syncytial cells. Small sebaceous glands are almost completely effaced by necrosis of the sebocytes. Diffusely throughout the dermis and epidermis, there are low to moderate numbers of neutrophils and lymphocytes, with fewer eosinophils and macrophages, and scattered leukocytoclastic debris. Throughout the dermis, multifocally veins and to a lesser extent arteries have mural-associated neutrophils, and/or walls replaced by smudgy eosinophilic debris (fibrinoid necrosis), with intramural and perivascular leukocytoclastic debris. Perivascular spindlecells and occasionally endothelial cells contain eosinophilic cytoplasmic inclusion bodies. Within the subcutis there are similar vascular changes to those described in the dermis, although frequently larger vessels are affected, resulting in marked expansion of the subcutaneous adipose tissue by haemorrhage, fibrin, and wispy eosinophilic material (oedema).
Contributor’s Morphologic Diagnosis:
Haired skin and subcutis, ventrum: Dermatitis, folliculitis and cellulitis, necrotising, severe, diffuse, with leukocytoclastic vasculitis, keratinocyte intracytoplasmic eosinophilic inclusion bodies, keratinocyte viral syncytia, and epithelial ballooning degeneration.
Contributor’s Comment:
This case demonstrates a systemic form of pox virus infection in cats, resulting in a clinical presentation of progressive marked cellulitis and necrotising dermatitis. This case lacked an observed primary cutaneous lesion or respiratory clinical changes, and prior to post-mortem examination, whilst a vasculitis was suspected, pox-viral related vasculitis was not a considered differential. On microscopic examination of the dermal and subcutaneous lesions, the frequent intracytoplasmic inclusion bodies were noted prompting qPCR for feline pox virus on stored frozen tissues. Feline poxvirus qPCR reveals moderately high levels of feline pox virus within the tissues, therefore pox viral infection is considered the cause of the necrotising process that has resulted in the clinical and histological features described in this case. Immunohistochemistry for pox virus demonstrated specific immunoreactivity within epithelial cells in affected areas. This cat had an extended post-mortem interval in cool storage, which is likely to have contributed to the poor preservation of some inflammatory cells.
Cow pox/cat pox virus is a large, enveloped, double-stranded DNA, cytocidal virus that is part of the Orthopoxviridae genus, within the Poxviridae family. The orthopox genus also includes ectromelia virus, monkeypox virus, smallpox virus (variola), vaccinia virus, and rabbitpox virus. Cowpox is endemic in Europe and Northern and Central Asia with a wide host range that includes people.7,17,32 Cowpox infection in most species results in localised dermal lesions or systemic disease that may be fatal. Wild rodents, especially bank voles (Clethrionmys glareolus), serve as the viral reservoir and infection of rodents, and subsequently secondary species such as humans and cats, varies seasonally with a peak in autumn and subsequently secondary transmission to cats.1,4
Cow pox is the most common poxviral infection of cats, with the common presentation involving the skin, often initially with a single primary lesion, which is assumed to be secondary to intradermal inoculation via a rodent bite.26 Systemic infections can occur following the viraemic phase, and include pyrexia and lethargy, and occasionally pneumonia.20,26 Feline cowpox infection is occasionally concurrent with other feline viruses, including viruses expected to cause immune dysfunction. At this stage the relationship between the clinical progression and severity of feline cowpox infection in cats co-infected with other viruses is not clear.8 FIV has been suggested to exacerbate feline cowpox infection, with larger and more persistent primary lesions, however up to 30% of cats infected with cowpox are also seropositive to FIV with no change in disease course.1 Feline herpesvirus and feline cowpox co-infection resulted in a necrotising pneumonia involving both viruses and a pox-viral dermatitis, however the cat successfully recovered with supportive treatment and antimicrobials.14 A case of a cat with cowpox and feline parvovirus infection resulting in a dermatitis, panniculitis and enteritis was not associated with more severe poxvirus-associated disease.24
The FIV/FeLV status of the cat in this case was not defined. Special stains did not highlight bacteria within the subcutis, however a secondary bacterial infection, as indicated by the positive culture of the oedema fluid, is considered likely. Rare areas of ulcerated skin were associated with mats of superficial fungal hyphae, and deeper invasion was not observed. For both secondary infections, a systemic role for either the agents or elaborated toxins is considered a possible co-factor in the severe presentation in this case.
A systemic form of feline cowpox, primarily presenting with severe dermal oedema is unusual. This presentation has been previously reported in a 4 year old male neutered domestic short haired cat that exhibited known hunting behaviours, and ultimately resulted in pox-viral associated neurological lesions and euthanasia of the cat.2 More localised dermal oedema was described in a series of five cats that presented with dermal plaques, oedema and hyperaemia affecting the hindlimbs,16 and two cases of localised forelimb oedema in a four-cat feline cowpox case series from the UK.9 Of the five-cat case series, all cats were geographically and temporarily grouped and in four of the cases, the cowpox viruses had an identical haemagglutinin gene sequence suggesting a common source of infection.16
Pathogenesis. Epidermal lesions are the result of viral replication in keratinocytes resulting in cellular dysfunction and eventual lysis either due to host-cell related mechanisms or to the actions of immune cells such as NK cells.3,30 Viral proteins involved in viral entry to cells also induce host cell fusion, resulting in syncytia formation.28 Cowpox, similar to other pox viruses, contains an epidermal growth factor homologue that is able to influence cellular differentiation and proliferation of keratinocytes.27 The virus can infect and damage endothelial cells and in some experimental models produces a consumptive coagulopathy, resulting in more widespread dermal or submucosal necrotising lesions as a consequence of vascular damage, thrombosis and tissue infarction.5,15,23 Cowpox virus has a range of immunomodulatory proteins that include IFN-gamma receptor and TNF-alpha receptor homologues, IL-1beta binding proteins, and anti-inflammatory Serpins.27
Typical and atypical clinical findings. Cats often present with a single primary skin lesion on the head, neck, forelimb or paws.1 Secondary bacterial infection of the lesion may induce confounding clinical signs. A viremia with pyrexia, lethargy and inappetence may follow 1-3 weeks following the initial infection.26 Widespread skin lesions may follow the viremia with papules and nodules that ulcerate and crust, but skin healing is ultimately expected.1 The mucocutaneous junction and oral cavity may be affected, forming ulcerative foci.9 Pneumonias in the absence of skin lesions has also been reported rarely with both cases resulting in a fatal pneumonia that was caused by a genetically similar but not identical viral strain.11,25 Exotic felids, especially cheetahs, can be infected with cowpox virus.18 The clinical signs range from dermal and oral lesions to a necrotising pneumonia, of which two out of nine cases (22%) of cheetah infections were fatal.31
Typical gross findings. The typical macroscopic epidermal changes include erythematous macules developing into papules and then pustules that rupture and crust.1 In this case, and in other reported atypical feline cowpox presentations, there was marked dermal and subcuticular oedema due to, in this case at least, widespread pox-viral associated vasculitis/vascular dysfunction. Marked subcutaneous oedema is a clinical feature more associated with Mousepox (ectromelia) virus infection.22
Typical microscopic findings. The typical microscopic findings in cases of feline cowpox are well defined. Epidermal lesions start with keratinocyte cytoplasmic swelling and vacuolation (intracellular oedema and ballooning degeneration), and progress through cell rupture to multiloculated vesicles (reticular degeneration) and epidermal necrosis. 1,12 Epidermal hyperplasia is often present and may be marked. Accompanying dermal changes include oedema, mononuclear cell infiltrate and a variable neutrophilic infiltrate. With time, the neutrophilic infiltrate extends into the epidermis creating pustules. Cox pox, like other pox viruses, creates intracytoplasmic inclusion bodies that vary in number and size. Large brightly eosinophilic inclusions are identified as A-type inclusions, and are predominantly composed of viral protein in which mature virions are embedded.6 A-type inclusions are thought to protect infectious particles after release into the environment, and increase in size throughout the infection by coalescence of multiple smaller bodies.13 B-Type pox inclusions are smaller, more basophilic, and are the sites of viral replication (viral factories).
Ultrastructure. On electron microscopy Poxviral particles are enveloped and are brick shaped. Single virions have multiple protruding surface spicules.11
Additional diagnostic tests. Confirmation of the diagnosis can be made using electron microscopy, immunofluorescence, virus isolation, and PCR on tissues, which also includes skin scabs and pulmonary or thoracic aspirates.21,26
Treatment. Treatment focuses on controlling/preventing secondary bacterial infections and immunomodulation with recombinant feline interferon omega.2,20
Contributing Institution:
Department of Veterinary Medicine
The Queen's Veterinary School Hospital University of Cambridge.
Cambridge CB3 0ES, UK.
https://www.vet.cam.ac.uk
JPC Diagnosis:
Haired skin and subcutis: Vasculitis, necrotizing, diffuse, severe, with cutaneous infarction, epithelial ballooning degeneration, numerous epithelial viral syncytia and intracytoplasmic inclusions.
JPC Comment: The contributor provides an excellent, thorough overview of cowpox infection in cats. While the cat is the most commonly recognized incidental host, cowpox
| Genus |
Representative Viruses |
|
Orthopoxvirus |
· Cowpox virus · Vaccinia virus (buffalopox virus, rabbitpox virus) · Horsepox virus · Camelpox virus · Ectromelia virus (mousepox virus) · Monkeypox virus |
|
Parapoxvirus |
· Orf virus (contagious pustular dermatitis virus, contagious ecthyma virus) · Pseudocowpox virus (milker's nodule virus) · Bovine papular stomatitis virus · Parapox virus of red deer |
|
Avipoxvirus |
· Fowlpox virus · Pigeonpox virus |
|
Capripoxvirus |
· Sheeppox virus · Goatpox virus · Lumpy skin disease virus |
|
Leporipoxvirus |
· Myxoma virus · Rabbit fibroma virus |
|
Suipoxvirus |
· Swinepox virus |
|
Molluscipoxvirus |
· Molluscum contagiosum virus |
|
Yatapoxvirus |
· Tanapox virus · Yaba monkey tumor virus |
|
Table 2-1. Selected Poxviridae genera and representative members. |
|
The Poxviridae is a large family of enveloped DNA viruses that are highly epitheliotropic and cause cutaneous and systemic disease.19 Poxviruses encode their own replication machinery, including an RNA polymerase, which enables their characteristic cytoplasmic replication. Animal poxviruses exist within the subfamily Chordopoxvirinae, which is further subdivided into 22 different genera (see Table 2-1 for a sampling of genera and representative members).
The orthopoxviruses, including cowpox, are notable for their wide host spectrum and are among the most important and well-characterized poxviruses due to their impact on human and animal health. Their most notable and feared member, the Variola virus, is the causative agent of smallpox.29
Poxviruses damage cells in multiple ways. Degenerative changes are caused by direct cytotoxic damage due to poxviral replication and subsequent rupturing of the host cell membrane, leading to typical vesicular lesions.19 As in this case, the dermis and submucosa may be subject to ischemia due to vascular damage caused by viral replication within endothelial cells.19 Poxviruses also induce proliferative lesions due, in some poxviruses, to a viral gene that encodes a mitogenic protein with significant similarity to epidermal growth factor.
Pox lesions tend to develop in a particular sequence, with erythematous macules becoming papular, and then vesicular.19 The vesicles become “pocks,” pustules with a depressed center and raised border which then rupture, forming crusts.19 Histologically, poxvirus lesions are characterized by marked epithelial hyperplasia with serocellular crusts and intracytoplasmic inclusion bodies. Both the gross and histologic lesions are characteristic, facilitating easy diagnosis in most cases.
In conference, participants discussed the remarkable coagulative necrosis present in the epithelium and dermis in this case. The moderator emphasized that this indicator of infarction, when present, should precipitate a close evaluation of deep vessels. While we noted the contributor’s description of cytoplasmic inclusion bodies within affected endothelium, no convincing endothelial cytoplasmic inclusions were found in the sections we received in this submission. The unrewarding hunt for endothelial inclusions did not dampen the participants’ enthusiasm for the robust, multifocal vasculitis, believed by the moderator to be the main driver of the striking pathology in this case; thus, the JPC morphologic diagnosis leads with vasculitis to reflect the centrality of this histologic finding.
References:
- Bennett M, Gaskell CJ, Baxby D, Gaskell RM, Kelly DF, Naidoo J. Feline cowpox virus infection. 1990. J Small Anim Pract. 1990;31:167-173.
- Breheny CR, Fox V, Tamborini A, et al. Novel characteristics identified in two cases of feline cowpox virus infection. JFMS Open Rep. 2017;3(2):205511-6917717191.
- Burshtyn DN. NK cells and poxvirus infection. Front Immunol. 2013;4:7.
- Chantrey J, Meyer H, Baxby D, et al. Cowpox: reservoir hosts and geographic range. Epidemiol Infect. 1999;122(3): 455-60.
- Chapman JL, Nichols DK, Martinez MJ, Raymond JW. Animal models of orthopoxvirus infection. Vet Pathol. 2010;47(5):852-70.
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31-124.
- Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010; 140(3-4):229-36.
- Foster AP. Immunomodulation and immunodeficiency. Vet Dermatol. 2004; 15(2):115-26.
- Godfrey DR, Blundell CJ, Essbauer S, et al. Unusual presentations of cowpox infection in cats. J Small Anim Pract. 2004;45(4):202-5.
- Greene CE. Poxvirus infections. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Elsevier; 2012:166-169.
- Hinrichs U, van de Poel H, van den Ingh TSGAM. Necrotizing Pneumonia in a Cat Caused by an Orthopox Virus. J Comp Path.1999;121(2):191-196.
- Hnilica K. Viral, Rickettsial, and Protozoal Skin Diseases. Small Animal Dermatology: A color atlas and therapeutic guide (Third Edition),B. Saunders; 2011:159-174.
- Howard AR, Moss B. Formation of orthopoxvirus cytoplasmic A-type inclusion bodies and embedding of virions are dynamic processes requiring microtubules. J Virol. 2012;86(10):5905-14.
- Johnson MS, Martin M, Stone B, Hetzel U, Kipar A. Survival of a cat with pneumonia due to cowpox virus and feline herpesvirus infection. J Small Anim Pract. 2009;50(9):498-502.
- Johnson RF, Yellayi S, Cann JA, et al. Cowpox virus infection of cynomolgus macaques as a model of hemorrhagic smallpox. Virology. 2011;418(2):102-12.
- Jungwirth N, Puff C, Köster K, et al. Atypical cowpox virus infection in a series of cats. J Comp Pathol. 2018; 158:71-76.
- Kaysser P, von Bomhard W, Dobrzykowski L, Meyer H. Genetic diversity of feline cowpox virus, Germany 2000-2008. Vet Microbiol. 2010;141(3-4):282-8.
- Marennikova SS, Maltseva NN, Korneeva VI, Garanina N. Outbreak of pox disease among carnivora (felidae) and edentata. J Infect Dis. 1977;135 (3):358-66.
- Mauldin EA, Peters-Kennedy J. Integumentary System. In: Maxie MG, ed. Pathology of Domestic Animals. Vol 1. 6th ed. Elsevier; 2016:616, 619-620.
- McInerney J, Papasouliotis K, Simpson K, et al. Pulmonary cowpox in cats: five cases. J Feline Med Surg. 2016;18(6): 518-25.
- Möstl K, Addie D, Belák S, et al. Cowpox virus infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15(7):557-9.
- Nicklas W, Bleich A, Mähler M. Viral Infections of Laboratory Mice. In: Hedrich HJ, Bullock G, eds. The Laboratory Mouse. Elsevier;2012:427-480.
- Roth SJ, Klopfleisch R, Osterrieder N, Tischer BK. Cowpox virus serpin CrmA is necessary but not sufficient for the red pock phenotype on chicken chorioallantoic membranes. Virus Res. 2012;163(1): 254-61.
- Schaudien D, Meyer H, Grunwald D, Janssen H, Wohlsein P. Concurrent infection of a cat with cowpox virus and feline parvovirus. J Comp Pathol. 2007;137(2-3):151-4.
- Schöniger S, Chan DL, Hollinshead M, Humm K, Smith GL, Beard PM. Cowpox virus pneumonia in a domestic cat in Great Britain. Vet Rec. 2007;160(15): 522-3.
- Scott DW, Miller WH Jr., Griffin CE. Viral, Rickettsial, and Protozoal Skin Diseases. In: Miller W, Griffin C, Campbell K, eds. Muller & Kirk's Small Animal Dermatology. Elsevier;2001:517-542.
- Seet BT, Johnston JB, Brunetti CR, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377-423.
- Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. Poxvirus multiprotein entry-fusion complex. Proc Natl Acad Sci. 2005;102(51):18572-7.
- Silva NIO, Silva de Oliveira J, Kroon EG, Trinidade G, Betania PD. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses. 2021;13(1):43.
- Smith GL, Law M. The exit of vaccinia virus from infected cells. Virus Res. 2004;106(2):189-97.
- Stagegaard J, Kurth A, Stern D, et al. Seasonal recurrence of cowpox virus outbreaks in captive cheetahs (Acinonyx jubatus). PLoS One. 2017;12(11): e0187089.
- Vorou RM, Papavassiliou VG, Pierroutsakos IN. Cowpox virus infection: an emerging health threat. Curr Opin Infect Dis. 2008;21(2):153-6.