Signalment:
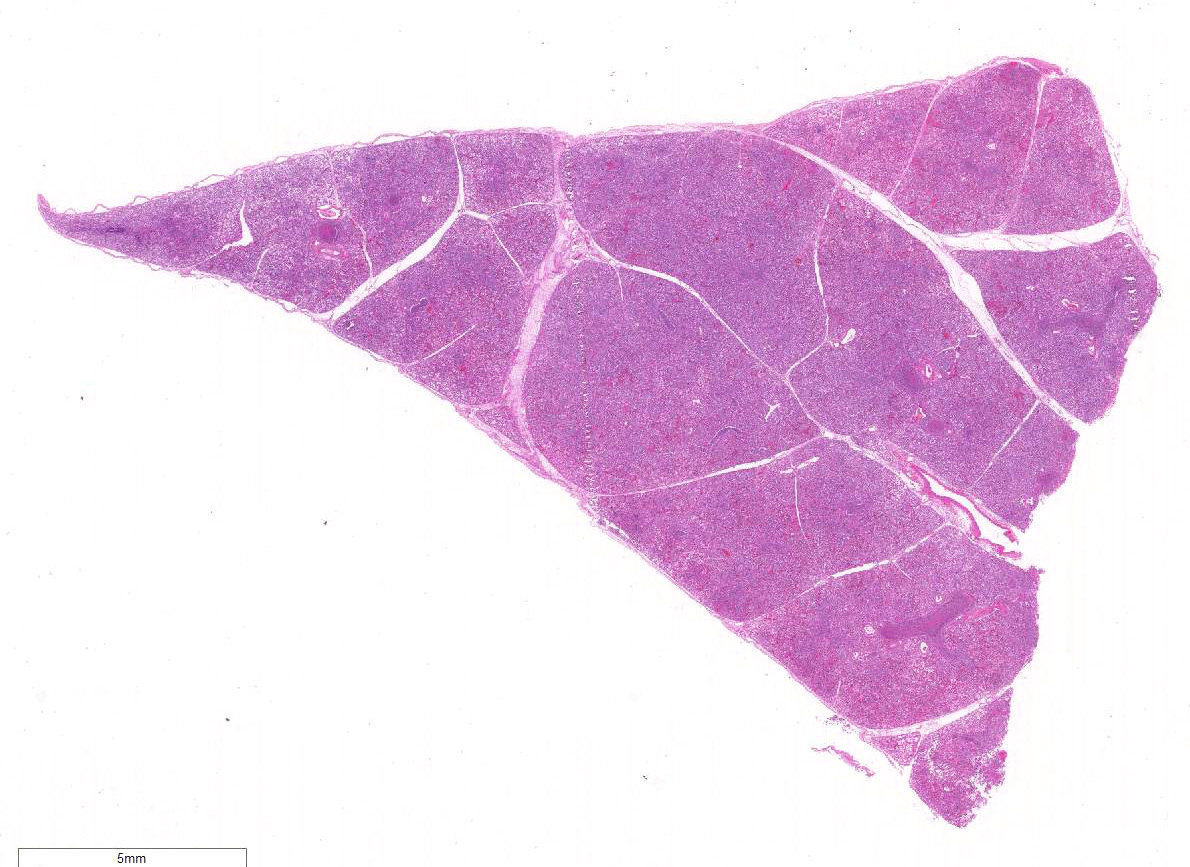
Gross Description:
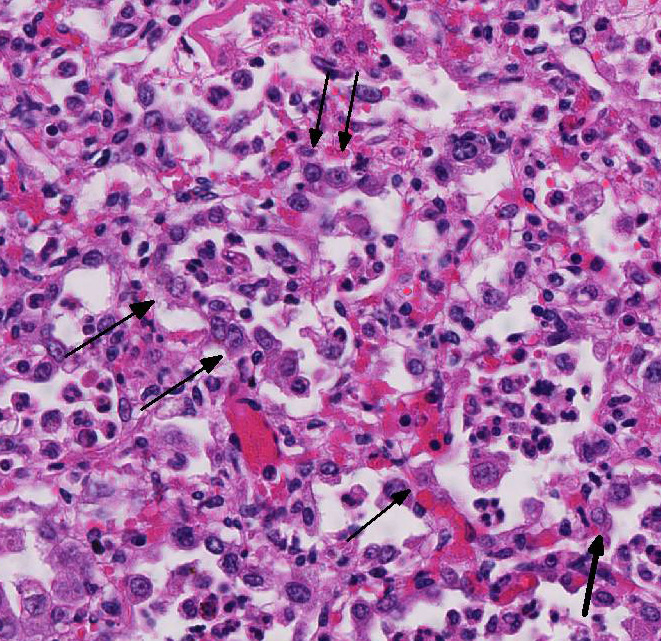
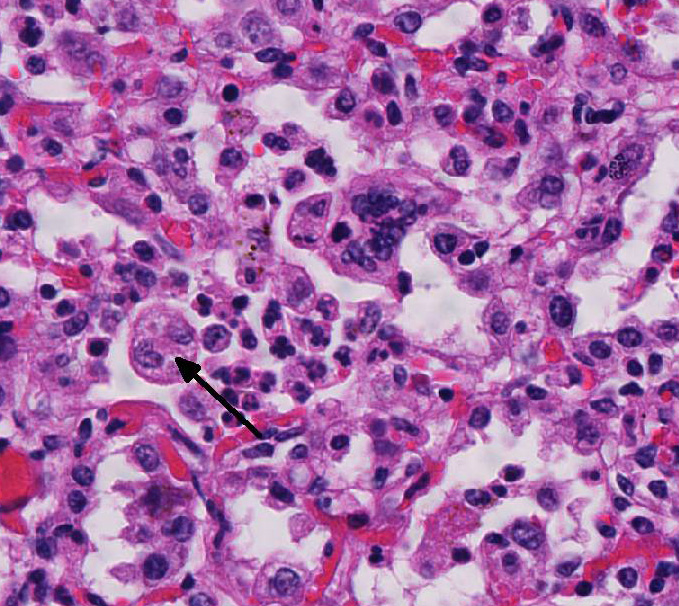
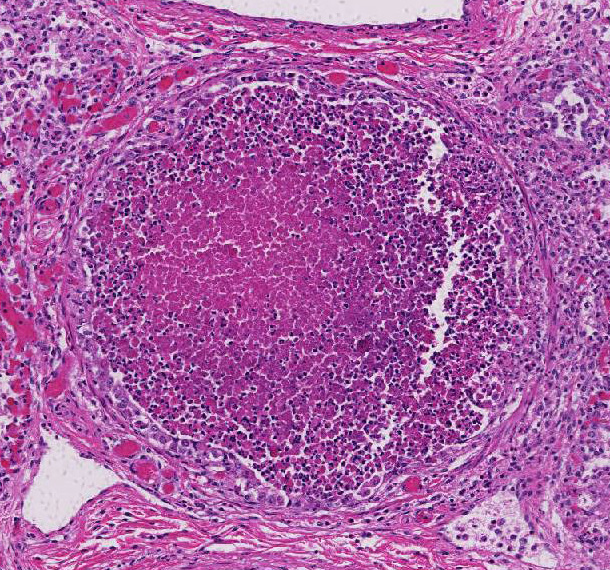
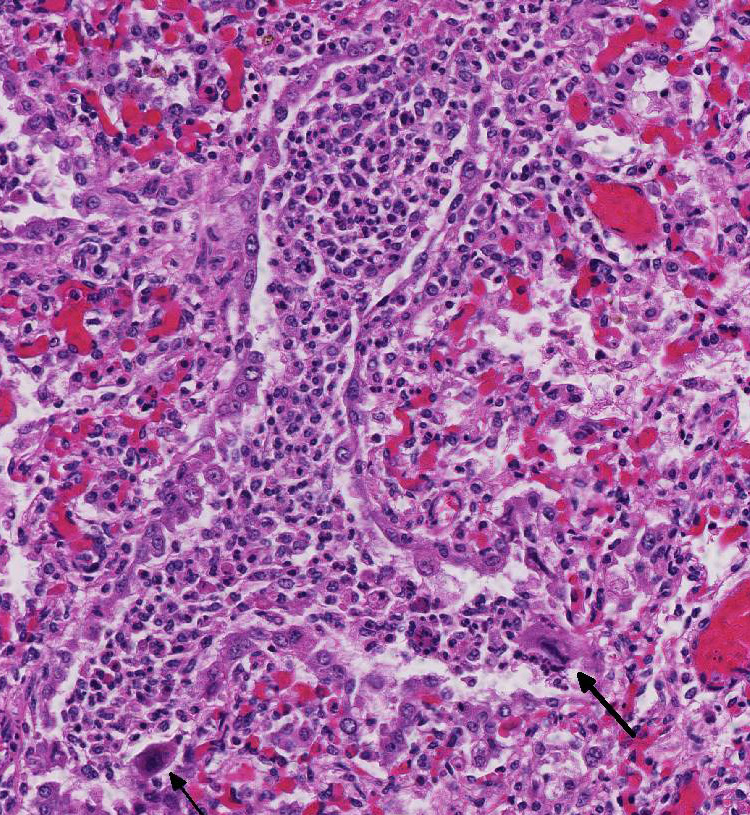
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
BRSV is a cause of enzootic pneumonia in 2-week to 5-month-old calves with a peak incidence at 1-3 months of age. BRSV can also cause fatal bronchointerstitial pneumonia in feedlot cattle but a role of BRSV in acute interstitial pneumonia in the late feeding period is questionable.1
BRSV is a member of the Pneumovirus genus in the family Paramyxoviridae. Experimental infections result in less severe clinical disease than natural cases, attributed to a reduction in virulence as a result of in vitro passage of the virus. In natural infections, viral antigen can be located in bronchiolar epithelium, type II pneumocytes, and macrophages, and less frequently in the nasal, tracheal, and bronchial epithelium.1
Gross lesions of natural BRSV infection differ in cranioventral and caudodorsal areas of the lung. The cranioventral lung is atelectatic, collapsed, deep red or mottled, and rubbery. In contrast, the caudodorsal areas fail to collapse and are edematous, heavy and firmer than normal. Variations of the gross lesions do occur, such that some cases may have a generalized rubbery texture with no difference between cranial and caudal lung, and in some cases there may be formation of bullae.1
Microscopic lesions of BRSV pneumonia include bronchointerstitial pneumonia with necrotizing bronchiolitis, formation of bronchiolar epithelial syncytia, and exudative or proliferative alveolitis. In acute lesions, bronchioles are lined by flattened epithelium, bronchiolar lumens contain necrotic epithelial cells and neutrophils. Alveoli contain neutrophils and macrophages; hyaline membranes are in-frequent. Syncytia may be difficult to distinguish from the multinucleate macro-phages that clear fibrin from alveoli in cases of fibrinous pneumonia, however, the presence of bronchiolar syncytia is a more reliable indicator of viral infection. Intracytoplasmic eosinophilic inclusion bodies are occasionally present in syncytial cells and uncommonly in bronchiolar and alveolar epithelium.1
BRSV infections in calves have many similarities to human RSV infections. Children vaccinated with formalin-killed RSV vaccine reportedly had more severe respiratory disease than those who were not vaccinated.3 Similarly, there are reports of exacerbated disease after vaccination of cattle with formalin-killed vaccine. Kalina et al5 showed that vaccination with formalin killed vaccine can enhance the disease, and attributed this to a shift to a Th2 immune response. Experimental infection of calves with BRSV, followed by infection with Histophilus somni resulted in an infection that was more severe than Histophilus alone; this was attributed to a shift to IgE production by the BRSV infection.4 Current trends are to develop vaccines for BRSV that shift the immune response to a Th1 response that will favor virus-killing mechanisms.
The calf in this study was co-infected with Mycoplasma bovis, and the necrotic cells and necrotic debris in the airways are attributed to M. bovis infection. The mycoplasmal infection in this calf probably is in early stages of development, since the necrotic lesions have not progressed beyond the airways. The early lesions of experimental M. bovis infection consist of suppurative exudates within small bronchioles, in which the leukocytes are stated to have a characteristic appearancethey are necrotic but retain their cellular outlines, and have hypereosinophilic cytoplasm, and unapparent or fragmented nuclei.1
JPC Diagnosis:
Conference Comment:
Like the contributor, some conference participants favored breaking the pattern of inflammation into two distinct processes. Participants argued that BRSV causes a fibrinous and proliferative interstitial pneumonia with a secondary suppurative and necrotizing bronchopneumonia caused by early co-infection with Mycoplasma bovis, detected by PCR in this case. Participants favoring the pattern of bronchointerstitial pneumonia countered that the virus causes the necrotizing lesions in the bronchi, bronchiolar epithelium, and type I pneumocytes, as well as thickening of the alveolar septae by infiltrates of leukocytes, and type II pneumocyte hyperplasia. They also point out that there is little evidence for typical M. bovislesions in this case. M. bovis classically causes a bronchopneumonia with distention of bronchioles and alveoli by a caseonecrotic exudate with an eosinophilic core, and are rimmed by ghost-like leukocytes remnants.1 Most conference participants agreed that those lesions associated with M. bovis are not a prominent feature of this case. Ultimately, participants agreed that the lesions in this case are characteristic for BRSV, which causes bronchointerstitial pneumonia in the cranio-ventral lung lobes.1,2,5
Conference participants also discussed additional causes of syncytial cells in the bovine lung, other than BRSV. Bovine parainfluenza virus 3 (BPIV-3), a member of the genus Respirovirus in the Paramyxoviridae family, can also induce the formation of syncytia with intracytoplasmic inclusion bodies.1 Similar to BRSV, another Paramyxoviridae family virus, BIPV-3 induces syncytial cell formation via a fusion (F) transmembrane glycoprotein.1,5 BIPV-3 usually causes mild bronchitis and bronchiolitis in cattle. Multinucleated alveolar macrophages can also resemble syncytial cells in cattle with fibrinous or granulomatous pneumonia. However, in BRSV, there would likely be both alveolar and bronchiolar syncytial cells.1
References:
2. Gershwin LJ. Bovine respiratory syncytial virus infection: immunopathogenic mechanisms. Anim Health Res Rev. 2007; 8:207-213.
3. Gershwin LJ, Berghaus LJ, Arnold K, Anderson ML, Corbeil LB. Immune mechanisms of pathogenetic synergy in concurrent bovine pulmonary infection with Haemophilus somnus and bovine respiratory syncytial virus. Vet Immunol Immunopathol. 2005; 107:119-130.
4. Kalina WV, Woolums AR, Berghaus RD, Gershwin LJ. Formalininactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine. 2004; 29:1465-1474.
5. Sacco R, McGill L, Pillatzki A, Palmer M, Ackermann M. Respiratory syncytial virus infection in cattle. Vet Pathol. 2014; 51:427-436.