Signalment:
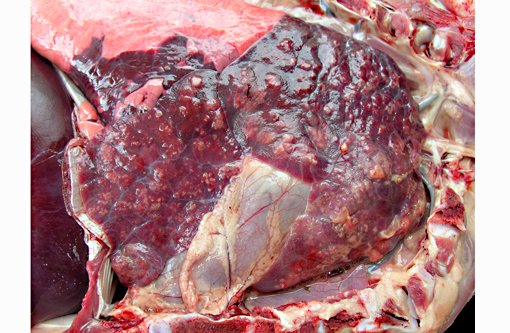
Gross Description:
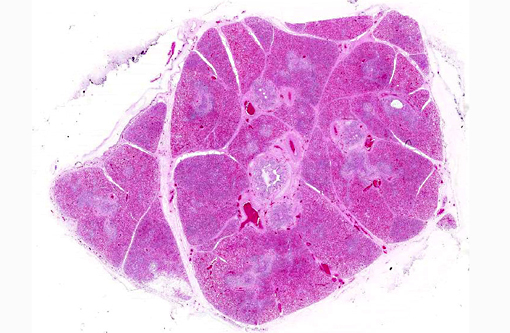
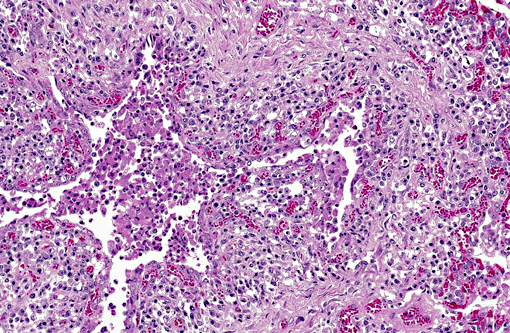
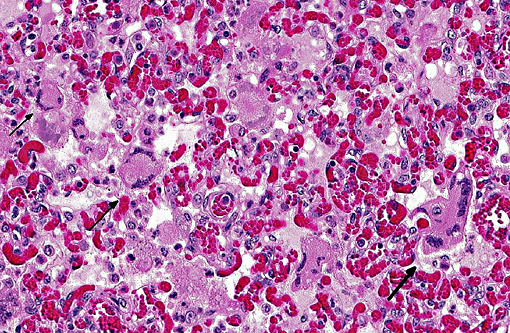
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Among those etiologic agents, bovine respiratory syncytial virus (BRSV) seems to be a major contributor to the bovine respiratory disease of BRD complex.(2) This virus belongs to the Pneumovirus genus within the subfamily Pneumovirinae, family Paramyxoviridae, and is an enveloped, non-segmented, negative-stranded RNA.(8) By electron microscopy, the morphology of the RSV virions appears to be either very pleomorphic, with a shape roughly rounded and a diameter between 150 and 35 nm, or filamentous with a length that can reach 5μm and a diameter between 60 and 100 nm.(10)
The bovine respiratory syncytial virus (BRSV) has been recognized as a pathogen in cattle responsible of an acute respiratory disease syndrome in beef and dairy calves since the early 1970s. The impact of BRSV infection on the cattle industry results in economic losses due to the morbidity, mortality, treatment and prevention costs that eventually lead to loss of production and reduced carcass value.(8) BRSV causes acute outbreaks of respiratory disease in 2-week to 5-month-old dairy and beef calves. This virus could also predispose to bacterial pneumonia in feedlot beef cattle and occasionally cause respiratory disease in naive adult dairy cows.(3)
The virus replicates predominantly in ciliated respiratory epithelial cells but also in type II pneumocytes. It appears to cause little or no cytopathology in ciliated epithelial cell cultures in vitro, suggesting that much of the pathology is due to the hosts response to virus infection. RSV infection induces an array of pro-inflammatory chemokines and cytokines that recruit neutrophils, macrophages and lymphocytes to the respiratory tract resulting in respiratory disease.(10)
Microscopic lesions in BRSV infections consist of bronchointerstitial pneumonia, characterized by necrotizing bronchiolitis, formation of bronchiolar epithelial syncytia, and exudative or proliferative alveolitis. The subacute lesions of BRSV represent early repair of the previous lesions and additional lymphocyte-mediated lysis of virus infected cells. Bronchiolitis obliterans may occur as early as 10 days after infection.(2,4)
JPC Diagnosis:
Conference Comment:
BRSV is one of many components of the commonly described bovine respiratory disease complex (BRDC), a pathologically complex and economically important disease which costs the U.S. cattle industry up to $1 billion per year.(8) While complex in its pathogenesis, the disease is ultimately a manifestation of the stress which cattle experience during weaning, processing, shipping and commingling. For this reason, it is logical that clinical signs of BRDC generally occur 7-10 days following this series of events resulting in the leading cause of morbidity and mortality in U.S. feedlots.(8)
BRSV is the largest player in the BRDC, with seroconversion estimates in some geographic areas of over 70% in calves under 12 months old.(8) With the addition of the aforementioned primary viral pathogens and the bacterial pathogens Mannheimia haemolytica, Bibersteinia trehalosi, Histophilus somni, Pasteurella multocida, Mycoplasma bovis and Trueperella pyogenes, assigning a specific etiologic diagnosis to gross or histopathologic lesions can prove to be an arduous task for the aspiring pathologist. Conference participants compared and contrasted the gross pathology of two other readily identifiable bacterial pathogens - M. haemolytica was described as resembling a brush fire histologically due to the advancing front of coagulative necrosis caused by bacterial toxins, while lesions of M. bovis often appear as golf balls due to its predilection for causing suppurative bronchiolitis with bronchiectasis.
A recent study compared the susceptibility of bovine airway epithelial cells to three different respiratory pathogens associated with the BRDC, with the differences between them perhaps due to direct correlation to the variation in disease manifestation. PI-3 readily entered the apical membrane of ciliated epithelial cells. These cells were largely resistant to BHV-1; however, when tight junctions were opened or the epithelial monolayer was damaged, BHV-1 infected basal cells. BRSV, in contrast, was unable to infect either epithelial or basal cells; however, the submucosal cells were susceptible though not specifically identified. The results led the authors to speculate on how BHV-1 and BRSV are able to cross the epithelial barrier as is apparently necessary to incite infection, even suggesting such bacterial pathogens as M. haemolytica are actually primary initiators.(6) This is in contrast with well-established data elsewhere of reduced ciliary clearance by epithelial degeneration and necrosis as a result of BRSV infections leading to secondary bacterial invasion.(3)
In large part thanks to its human relative, RSV, much has been described in regard to the immunopathogenic mechanisms of BRSV. The nonstructural proteins of RSV, NS1 and NS2, are instrumental in mediating resistance to IFN-stimulated responses, specifically by blocking phosphorylation and activation of IRF-3.(8) This interruption of the MyD88-independent pathway, which occurs exclusively through TLR 3 or TLR 4 signaling, reduces activated interferon thus limiting the innate immune response. While TLR 3 recognition of doublestranded, viral RNA utilizes only MyD88-independent signaling,(1) TLR 4 has the additional option of mediating NF-κB activation in concert with MD2 and CD14 when it recognizes the F protein of BRSV.(8) It is this fusion protein that is also responsible for mediating fusion of the viral envelope and the formation of viral syncytia seen histologically in both BRSV and PI-3 cases. The persistence of the virus in the face of widespread vaccination and in spite of the advances in the understanding of its pathogenic mechanisms has led to researchers developing improved technologies. Some promising examples include live attenuated vaccines which are devoid of either NS1 or NS2 and are capable of inducing robust antibody responses.(8)
Given the endemicity of these prominent respiratory pathogens and their considerable economic impact, significant opportunity for discovery remains in the complex pathogenesis of BRDC; though we leave the reader to speculate where in the process of beef production the most impactful of those opportunities lie, whether it is management considerations, preventive therapy, or treatment.
References:
1. Ackermann MR. Inflammation and healing. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:111-112.
2. Brodersen BW. Bovine respiratory syncytial virus. Vet Clin North Am Food Anim Pract 2010;26(2):323333.
3. Caswell JL. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet Pathol. 2014;51(2):393-409.
4. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Saunders Elsevier; 2007:596-598.
5. Fulton RW, Blood KS, Panciera RJ, et al. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J Vet Diagn Invest. 2009;21:464-477.
6. Kirchhoff J, Uhlenbruck S, Goris K, Keil GM, Herrler G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet Res. 2014;45:20.
7. Panciera RJ, Confer AW. Pathogenesis and pathology of bovine pneumonia. Vet Clin North Am Food Anim Pract. 2010; 26(2):191214.
8. Sacco RE, McGill JL, Pillatzki AE, Palmer MV, Ackermann MR. Respiratory syncytial virus infection in cattle. Vet Pathol. 2014;51(2):427-436.
9. Sarmiento-Silva RE, Nakamura-Lopez Y, Vaughan G. Epidemiology, molecular epidemiology and evolution of bovine respiratory syncytial virus. Viruses. 2012;4:3452-3467.
10. Valarcher J-F, Taylor G. Bovine respiratory syncytial virus infection. Vet Res. 2007;38:153180.