Signalment:
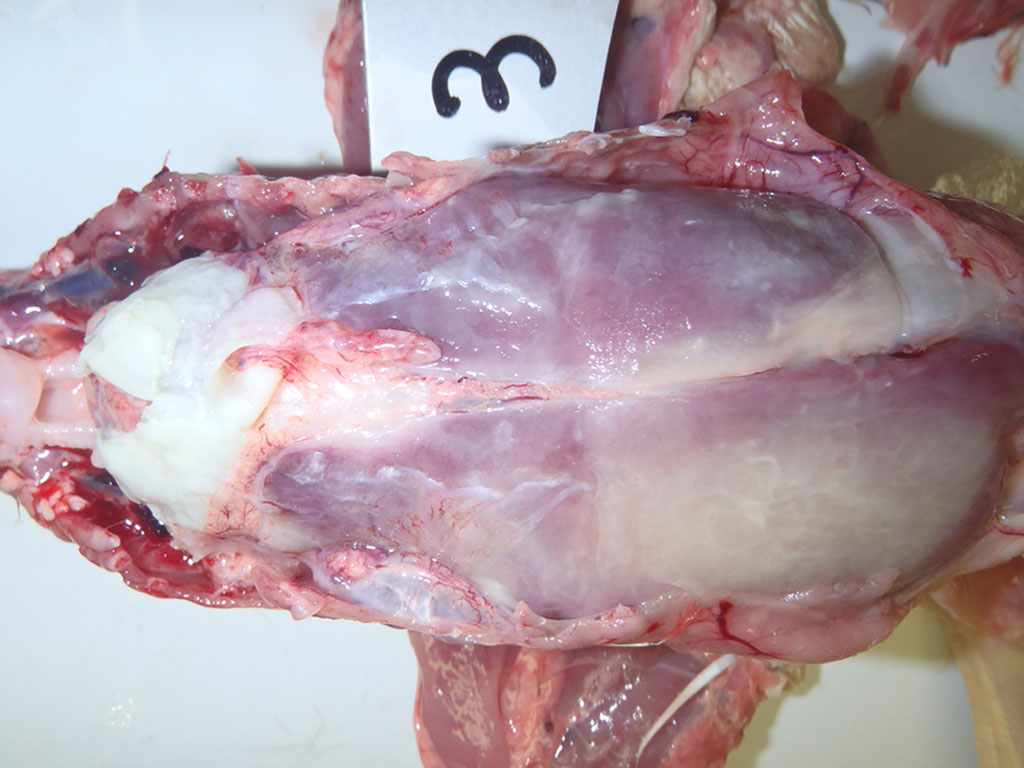
Gross Description:
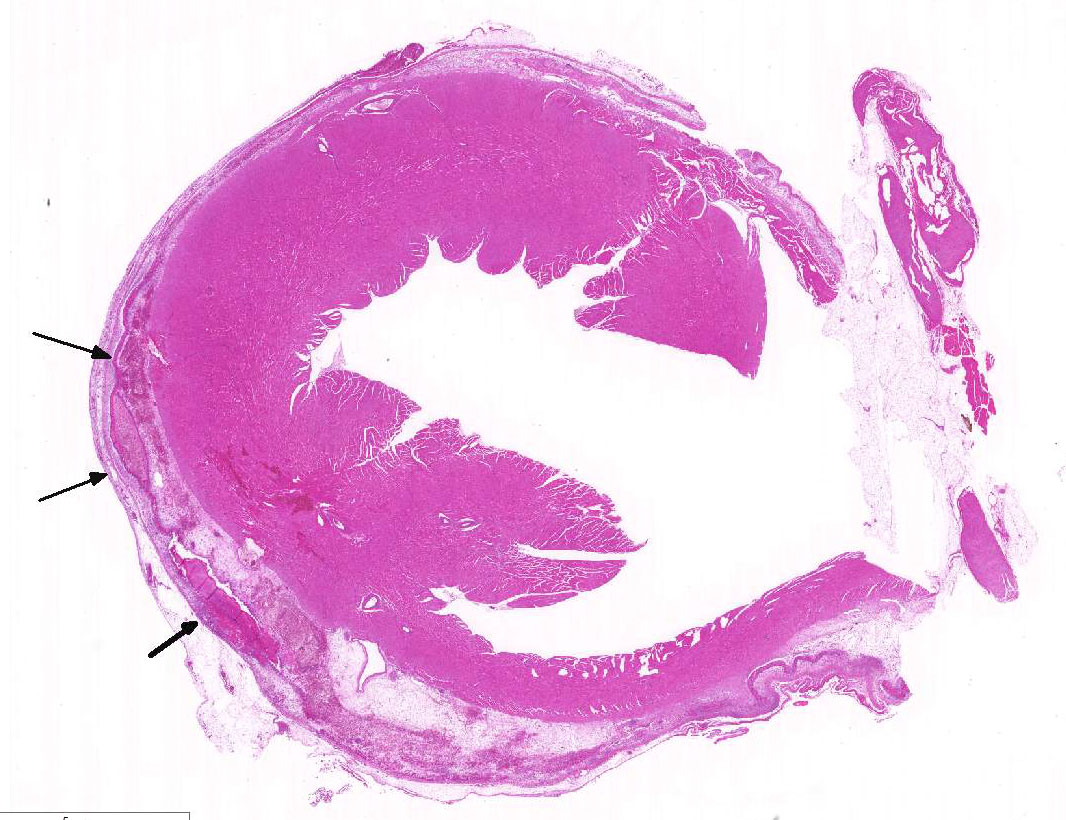
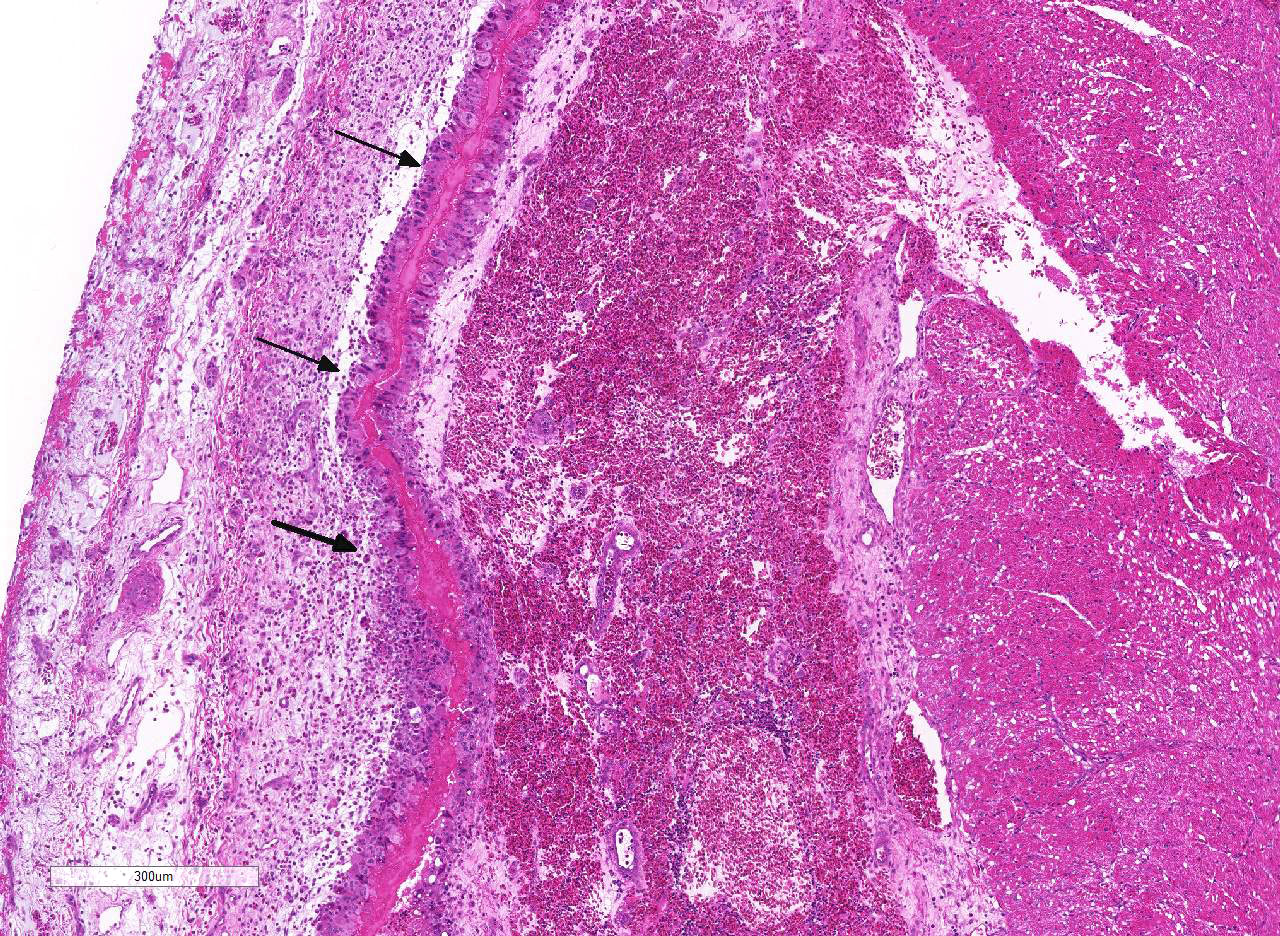
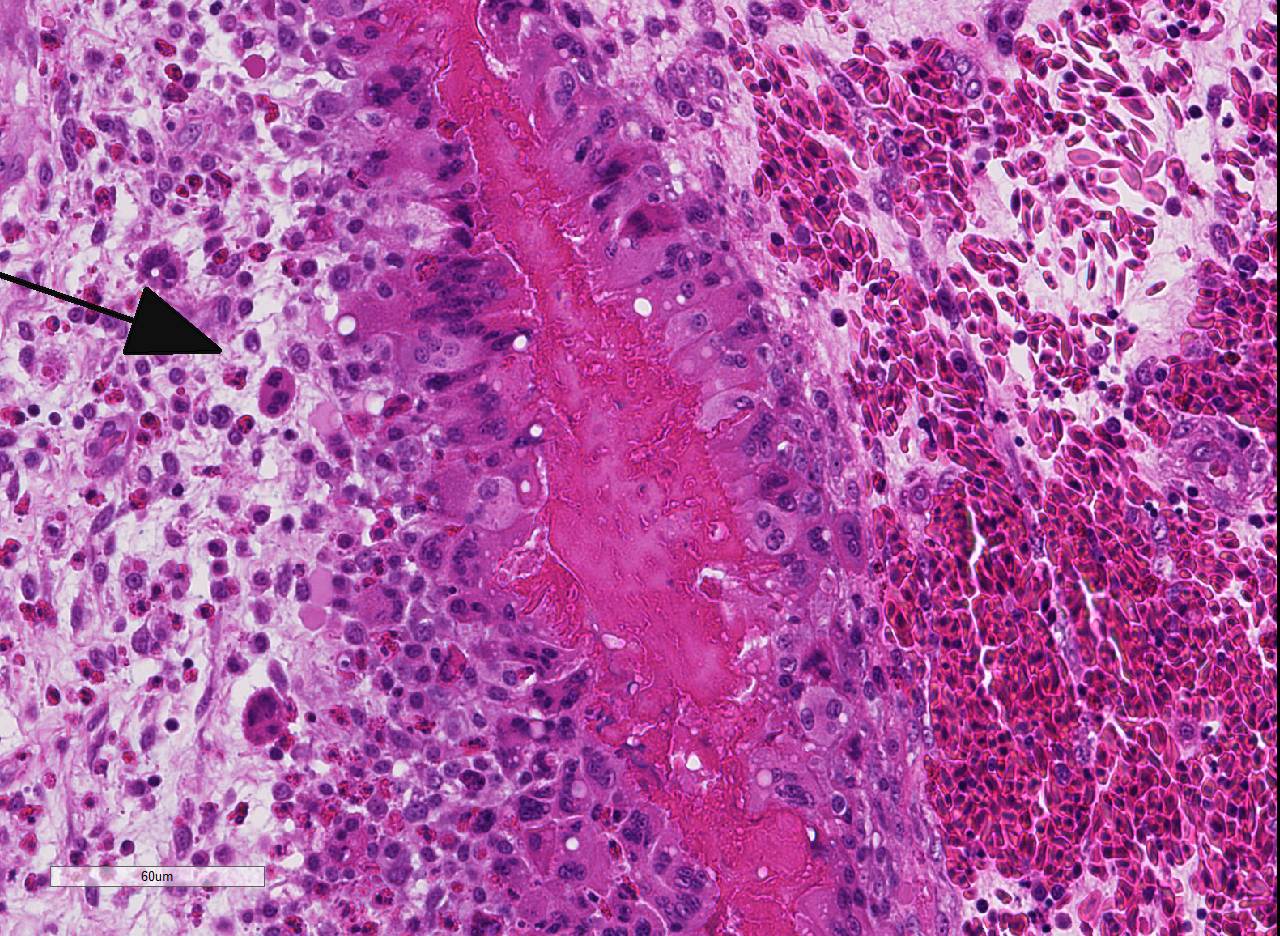
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Characteristic pathological findings, such as pericarditis and perihepatitis observed in the present case, are highly suggestive of RA infection. However, a definitive diagnosis of RA infection requires isolation and identification of RA from ducks suspected to be infected.9,10,14 There are difficulties associated with the identification of RA.9,10 Riemerella anatipestifer is characterized by the absence of species-specific biochemical properties.5 Genetic sequencing of bacterial 16S ribosomal RNA and matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry are currently considered useful for the identification of RA.4,8,13,16
Other bacterial infections such as colibacillosis may cause gross lesions similar to those seen in RA-infected ducks.6 The differential diagnosis also include salmonellosis, pasteurellosis, streptococcosis, and Coenonia anatina infection.14
JPC Diagnosis:
Conference Comment:
As mentioned by the contributor, fibrinous pericarditis and epicarditis are the most characteristic lesions of RA. This case represents the chronic form of the disease, with progression from fibrinous to granulomatous epicarditis with abundant granulation tissue on the epicardial surface. The pericardium was likely removed prior to tissue processing and is not present in the microscopic sections submitted for evaluation.
Many conference participants had little experience with this entity histologically, which led to a discussion of the differential diagnosis for epicarditis and polyserositis in avian species. While not a typical presentation for Pasteurella multocida, fowl cholera can cause similar lesions in poultry, chickens, and waterfowl. 4 Salmonella pullorum causes peritonitis and death in hatchling chicks; and peritonitis, arthritis, and pericarditis in adults, and is often characterized by large hetrophilic granulomas in the myocardium. Escherichia coli and other coliform infections also can lead to fibrinous and heterophilic rmyocarditis, and should be considered as a differential in most avian species.4 Coenonia anatine, described by the contributor and briefly discussed during conference, also causes exudative serositis in ducks and geese.12Riemerella columbina causes similar disease as RA in pigeons.12 While not mentioned during conference, West Nile Virus, an arbovirus of the family Flaviviridae, should also be considered as a differential for necrotizing myocarditis of many avian species, with young chickens and geese being most likely to develop clinical disease and mortality. 1
Participants also discussed the three main rule-outs for polyserositis, pneumonia, and polyarthritis in pigs; among these include Haemophilus parasuis, Mycoplasma hyorhinis, and Streptococcus suis. 5 In horses, the most likely etiologies for polyserositis are Streptococcus equi and Streptococcus zooepidemicus. 2
References:
2. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:578.
3. Cha SY, Seo HS, Wei B, Kang M, Roh JH, Yoon RH, Kim JH, Jang HK. Surveillance and characterization of Riemerella anatipestifer from wild birds in South Korea. J Wildl Dis. 2015; 51:341-347.
4. Christensen H, Bisgaard M. Phylogenetic relationships of Riemerella anatipestifer serovars and related taxa and an evaluation of specific PCR tests reported for R. anatipestifer. J Appl Microbiol. 2010; 108:1612-1619.
5. Christensen JP, Bojesen AM, Bisgaard M. Fowl Cholera. In: Pattison M, McMullin PF, Bradbury JM, Alexander DJ, eds. Poultry Diseases. 6th ed. Philadelphia, PA: Saunders Elsevier; 2008:149-154.
6. Craig LE, Dittner KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:151- 152.
7. Glünder G, Hinz KH. Isolation of Moraxella anatipestifer from embryonated goose eggs. Avian Pathol. 1989; 18:351-355.
8. Hess C, Enichlmayr H, JandreskiCvetkovic D, Liebhart D, Bilic I, Hess M. Riemerella anatipestifer outbreaks in commercial goose flocks and identification of isolates by MALDI-TOF mass spectrometry. Avian Pathol. 2013; 42:151-156.
9. Hinz KH, Ryll M, Kohler B, Glunder G. Phenotypic characteristics of Riemerella anatipestifer and similar micro-organisms from various hosts. Avian Pathol. 1989; 27:33-42.
10. Leibovitz L. A survey of the socalled "anatipestifer syndrome". Avian Dis. 1972; 16:836-851.
11. Mavromatis K, Lu M, Misra M, Lapidus A, et al. Complete genome sequence of Riemerella anatipestifer type strain (ATCC 11845). Stand Genomic Sci. 2011; 4:144-153.
12. Pathanasophon P, Phuektes P, Tanticharoenyos T, Narongsak W, Sawada T. A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathol. 2002 31:267-270.
13. Rubbenstroth D, Ryll M, Knobloch JK, Köhler B, Rautenschlein S. Evaluation of different diagnostic tools for the detection and identification of Riemerella anatipestifer. Avian Pathol. 2013; 42:17-26.
14. Ruiz JA, Sandhu T. Riemerella anatipestifer infection. In: Swayne DE, ed. Disease of poultry, 13th ed. Ames, IA; Wiley Blackwell publishing, 2013:823-828.
15. Ryll M, Christensen H, Bisgaard M, Christensen JP, Hinz KH, Köhler B. Studies on the prevalence of Riemerella anatipestifer in the upper respiratory tract of clinically healthy ducklings and characterization of untypable strains. J Vet Med B Infect Dis Vet Public Health. 2001; 48:537- 546.
16. Tsai HJ, Liu YT, Tseng CS, Pan MJ. Genetic variation of the ompA and 16S rRNA genes of Riemerella anatipestifer. Avian Pathol. 2005; 34: 55-64.