Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 5
September 27th, 2017
CASE I: S17-3588 (JPC 4100988).
Signalment: 8-week-old, male, breed not specified, Sus scrofa domesticus, porcine.
History: A piglet from an intensive pig farm, where a mortality rate increase of approximately 10% was registered in weaned piglets within a short time span, was submitted for necropsy. Affected animals displayed diarrhea that was occasionally bloody. All suckling piglets in this farm were vaccinated against porcine circovirus type 2 (PCV-2) and Lawsonia intracellularis.
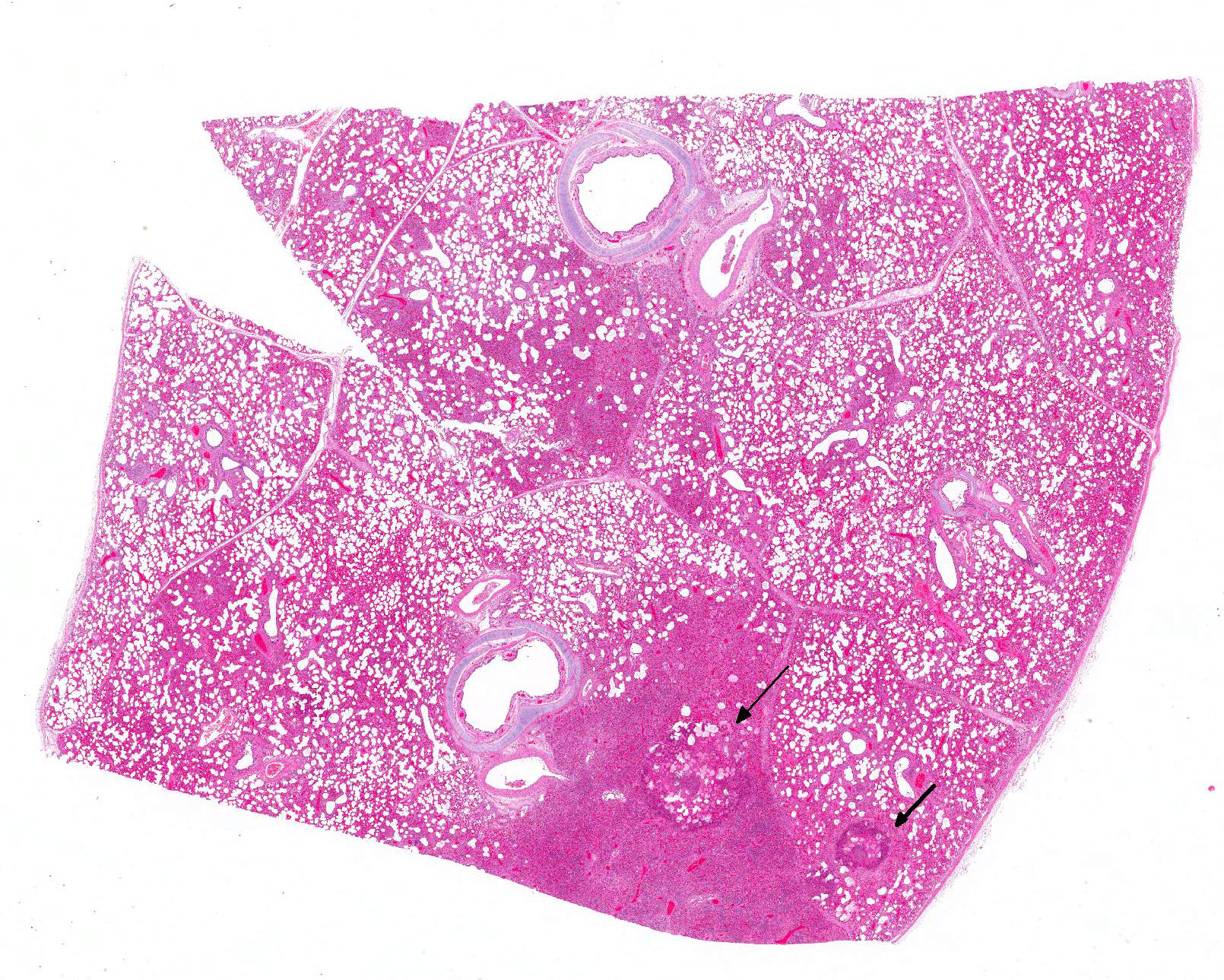
Gross Pathology: The piglet was severely emaciated and moderately dehydrated and its hind limbs were soiled with dry, dark brown feces. The cranial lobes of the lung were bilaterally dark red and with a firm consistency, whereas the rest of the lungs were bilaterally diffusely firm, heavy and did not collapse after the thorax was opened. The intestine was filled with a small amount of brown, liquid to creamy ingesta. No further gross lesions were detected in other organs.
Laboratory results:
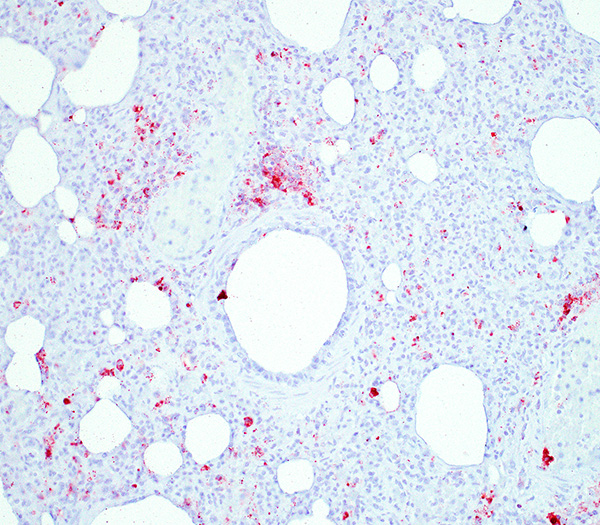
PCV-2 antigen immunohistochemical detection: High amount of PCV-2 antigen visible within the macrophages in the lung and lymphoid organs (spleen, Peyer´s patches, retroperitoneal and mesenteric lymph nodes, tonsils).
Bacteriology lung: High content Pasteurella multocida; moderate content Streptococcus suis.
Bacteriology intestinal content: Inconclusive (mixed microbial flora detection).
Serology: No antibodies against porcine respiratory and reproductive syndrome (PRRS) virus and classical swine fever (CSF) virus detected.
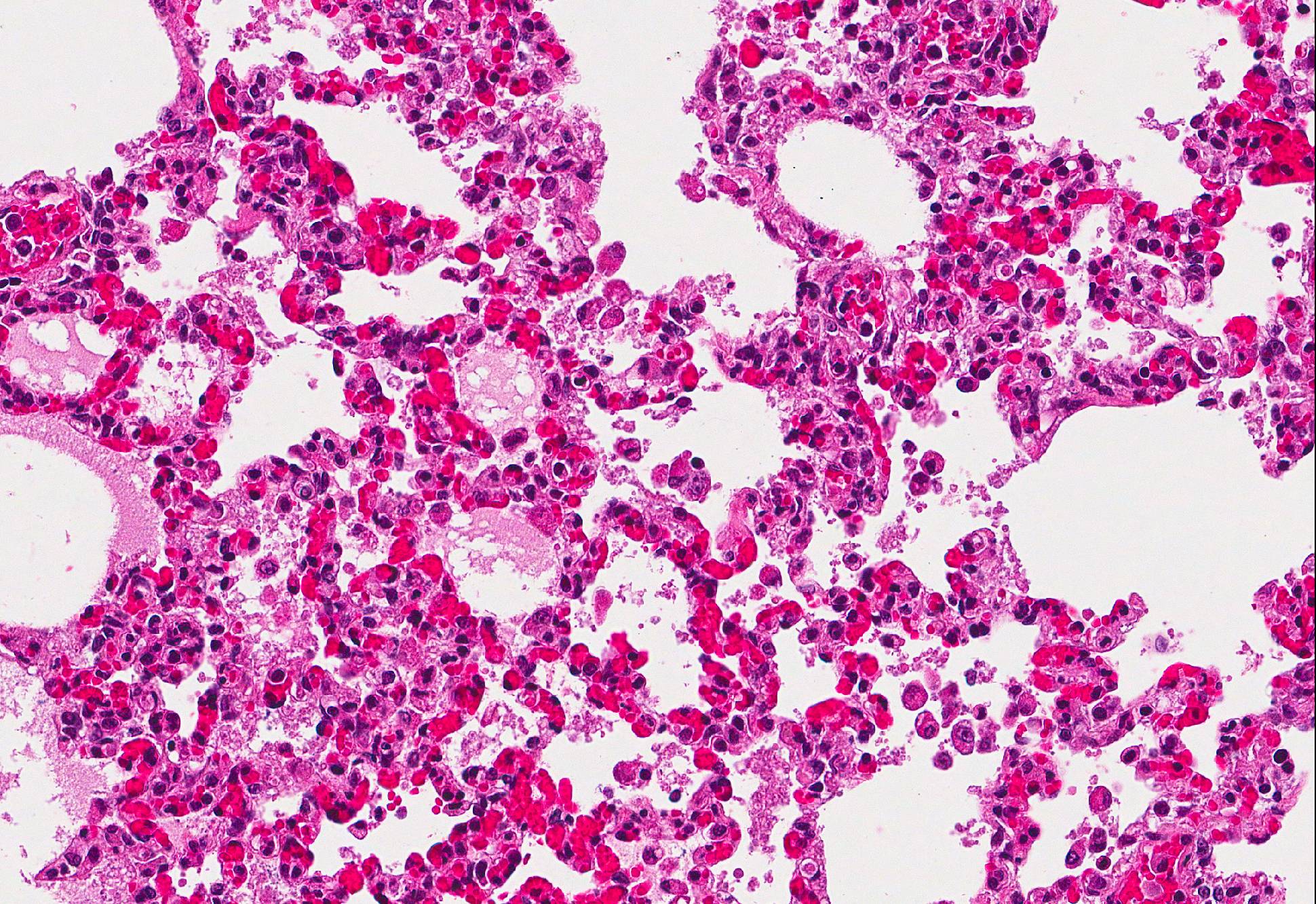
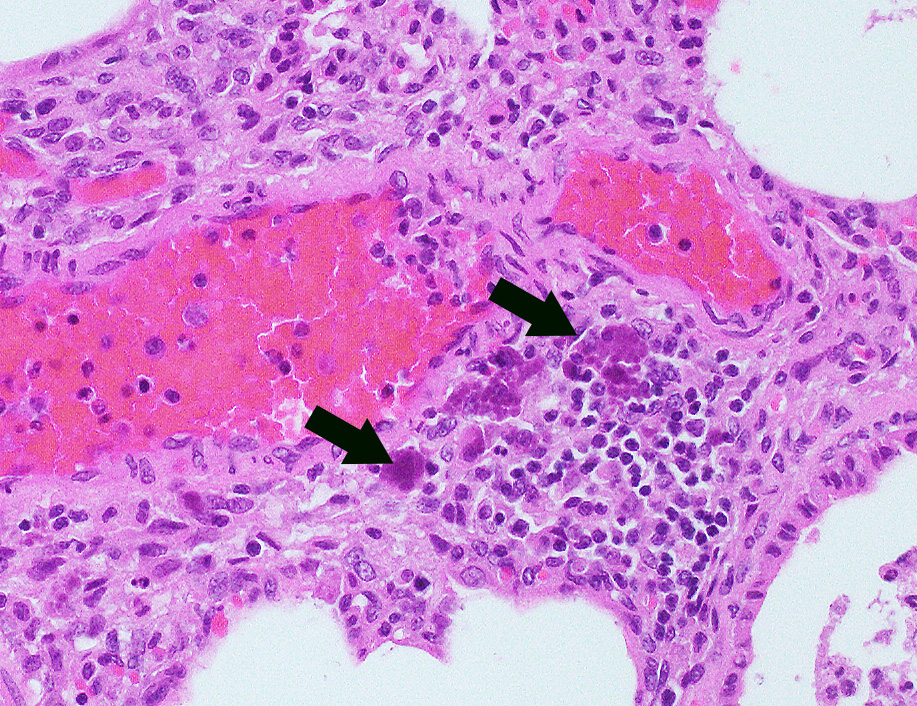
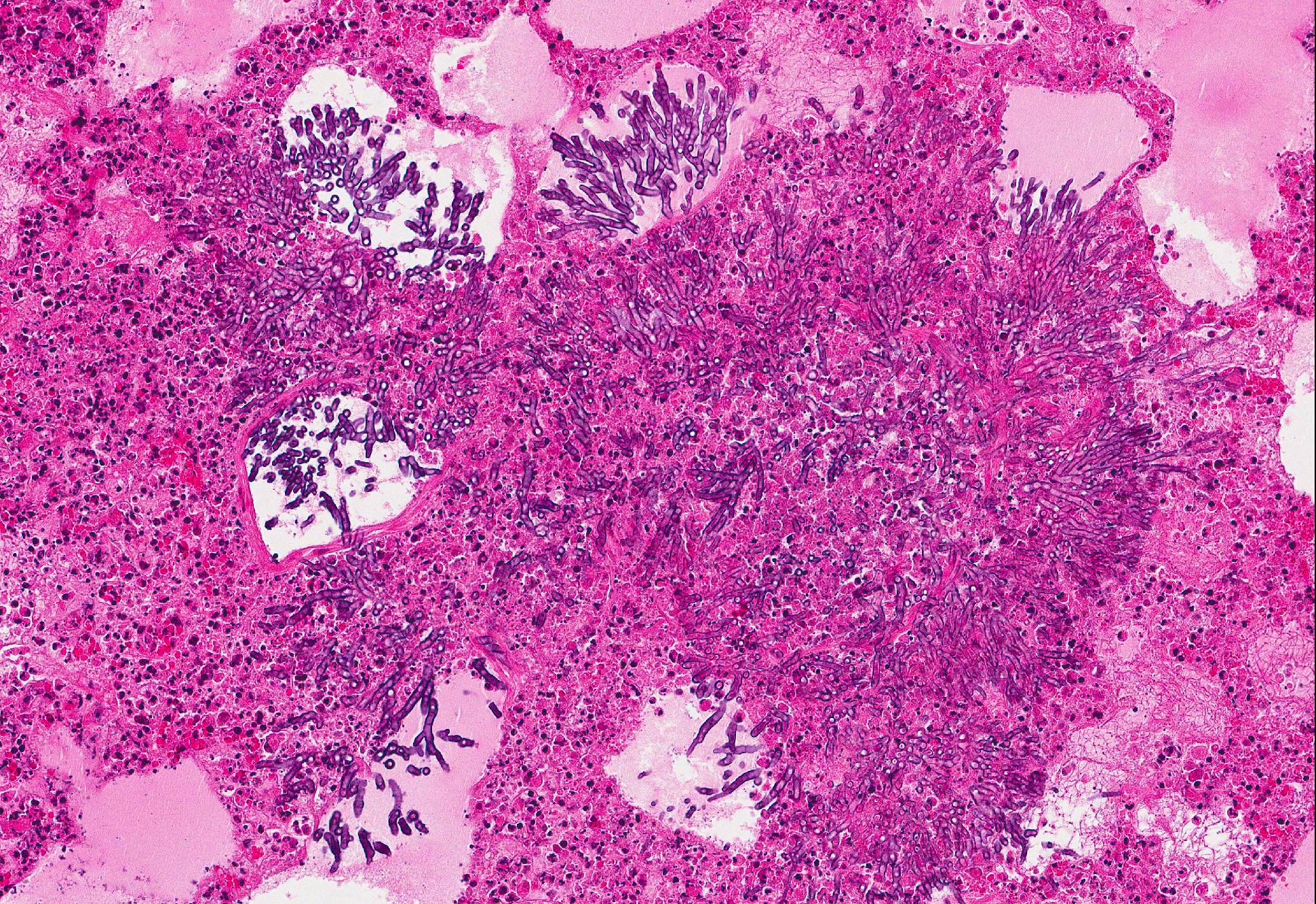
Microscopic Description: Lung: In approximately 90% of the parenchyma the alveolar septae are diffusely congested and multifocally severely thickened due to the presence of a high number of macrophages, lymphocytes and plasma cells, as well as fewer multinuclear giant cells of the foreign body type with up to five nuclei, neutrophils and accumulation of eosinophilic, foamy material (edema). The bronchus associated lymphoid tissue (BALT) is severely depleted and infiltrated by macrophages displaying moderate numbers of intracytoplasmatic, basophilic botryoid inclusion bodies. The bronchiolar epithelium is multifocally severely disrupted and the bronchiolar lumen is filled with a high amount of degenerated neutrophils intermingled with desquamated respiratory epithelium and occasional multinuclear giant cells. The surrounding alveoli are filled with high numbers of viable neutrophils and increased amounts of alveolar macrophages with a foamy cytoplasm, as well as a moderate number of free erythrocytes (hemorrhage) and edema. In most of the slides there are focal-extensive, hypereosinophilic areas displaying loss of cellular detail, karyorrhexis, karyolysis and pyknosis (lytic necrosis) associated with multiple, non-pigmented, septated fungal hyphae with 5-8 µm thick parallel walls and an acute angle dichotomous branching. Surrounding these necrotic areas there is a prominent granulomatous reaction characterized by the presence of high numbers of degenerate and viable neutrophils, epithelioid macrophages, lymphocytes and occasionally fibroblasts and sparse collagen proliferation. The interstitial septae and the pleura are diffusely moderately edematous.
Contributors Morphologic Diagnosis:
Lung: 1. Severe, diffuse, chronic, granulomatous, bronchointerstitial pneumonia with intralesional intracytoplasmatic botryoid basophilic inclusion bodies
2. Moderate, multifocal, acute, suppurative bronchopneumonia
3. Mild, focal-extensive to focal, chronic, granulomatous and necrotizing pneumonia with intralesional fungal hyphae (some slides only)
Contributors Comment: The postmortem and histological findings are compatible with an infection with porcine circovirus type 2 (PCV-2).
PCV are small non-enveloped, single-stranded DNA viruses with a circular genome that belong to the genus Circovirus from the Circoviridae family; two genotypes (PCV-1 and PCV-2) are currently described. While PCV-1 is nonpathogenic, PCV-2 is highly virulent and was isolated for the first time in 1997 in association with postweaning multisystemic wasting syndrome (PMWS), an important, globally present swine disease of great economic impact.1,6 Since several other often overlapping clinical syndromes were linked to PCV-2 infection in several age groups following its discovery, namely porcine respiratory disease complex (PRDC), porcine dermatitis and nephropathy syndrome (PDNS), enteric disease, reproductive failure and, more recently, PCV-2-associated cerebellar vasculitis, the term PCV-associated diseases (PCVADs) is nowadays used.1,5,6 The most common clinical presentation is systemic PCV-2 infection (PMWS) associated with PRDC-associated pneumonia.5 Other swine disease entities such as proliferative and necrotizing pneumonia, sow abortion and mortality syndrome, congenital hypomyelination and abortion with fetal myocarditis may also be associated to PCV-2 infection, but definite proof is still lacking.1
PCV-2 infection is usually slow progressing1 and infection occurs following inhalation or ingestion of viral particles present in contaminated oronasal-pharyngeal body fluids, feces and urine. Establishment of lymphoid tissue infection in the tonsils and Peyers patches is achieved through infection of mucosal dendritic cells, macrophages and lymphocytes, although the mechanisms that allow the virus to penetrate past the epithelial body barriers have yet to be fully elucidated. While macrophages are non-permissive to virus replication and act primarily as viral carriers within the organism, lymphocytes allow PCV-2 replication. Viral replication and release is associated with lymphocyte injury and lysis, which leads to severe lymphoid depletion and subsequent immunosuppression.10
Piglets between 5 and 12 weeks of age are usually most affected and display strong growth impairment or wasting, as well as a wide range of unspecific clinical signs, namely tachypnea, respiratory distress, fever, diarrhea, pallor, jaundice and central nervous signs. Sudden death can also occur. Morbidity is usually around 5-10% and affected animals usually die or are euthanized due to poor prognosis.1,6 The most commonly PMWS-associated lesions at necropsy include poor body condition, enlarged or atrophic lymph nodes, and interstitial pneumonia, very often associated with cranioventral bronchopneumonia.1,5 Histologically, the most commonly found lesions include severe lymphoid depletion associated with granulomatous lymphadenitis, bronchointerstitial pneumonia, granulomatous enteritis, interstitial nephritis, meningoencephalitis, and vasculitis. Sharply demarcated, single or in grapelike clusters (botryoid) arranged, basophilic inclusion bodies can be observed in the cytoplasma of macrophages of the lymphoid system, although their presence in other locations is also described.1,6
Besides the above-described lung lesions of the piglet, a severe, diffuse depletion of lymph follicles associated with a severe infiltration of macrophages displaying abundant intracytoplasmatic, botryoid, basophilic inclusion bodies in the tonsils, the spleen, Peyers patches, and in the retroperitoneal and mesenterial lymph nodes was observed. A high amount of PCV-2 antigen could be detected via immunohistochemistry both in the lymphoid organs and in the lung, thus confirming the PMWS diagnosis. Although PCV-2-associated lymphoid depletion does not always correlate with clinical disease and has also been described in subclinically infected pigs6, it is most likely that in the present case the PCV-2 infection led to a severe immunosuppression, which allowed the infection with opportunistic bacterial (Pasteurella multocida and Streptococcus suis) and fungal (most likely Aspergillus sp.) pathogens.
Interestingly, this piglet came from a farm where vaccination against PCV-2 was performed in suckling piglets. Several highly effective vaccines against PCV-2 are currently available on the market and have been widely used since 2006, which subsequently caused a reduction of the PCVAD disease prevalence;2,4,6,7,8,9 however, occasional outbreaks in vaccinated animals have been described.3 Even if PCV-2 infection alone is known to be sufficient to cause PMWS1, it is quite likely that host, management, co-infections (namely with parvovirus or PRRS virus) or immunostimulation play a crucial role in disease progression to PCVAD.1,7 In the present case the piglet was serologically negative for anti-PRRS virus antibodies and there was no evidence of a co-infection with other major swine pathogens. Therefore, an individual immune impairment or an individual vaccination failure due to an insufficient vaccination dosage may explain the above-described findings in the piglet.
JPC Diagnosis: 1. Lung: Pneumonia, interstitial, necrotizing, diffuse, moderate with diffuse, severe, bronchoalveolar-associated lymphoid tissue (BALT) depletion, rare intrahistiocytic botryoid inclusions, multinucleated macrophages, and intra-alveolar fungal cysts (etiology consistent with Pneumocystis sp.), breed not specified, porcine.
2. Lung: Bronchopneumonia, suppurative and histiocytic, multifocal to coalescing, moderate with intra and extracellular bacilli.
3. Lung: Arteritis, necrotizing, focally extensive, severe with intraluminal fungal hyphae.
Conference Comment: This case provides a slide that is ripe with descriptive features. Participants identified the same key features as the contributor: viral interstitial pneumonia, bacterial bronchopneumonia, and intralesional fungi.
There were very few examples of botryoid intracytoplasmic viral inclusions in macrophages present within depleted BALT tissues. The moderator noted that inclusions are seen less frequently in more recent cases, which has been described but not explained in the literature. The contributors submitted images were reviewed and discussed, including immunohistochemistry for PCV-2. We thank the contributor for providing additional images, which adds to the teaching/learning value of the case.
Conference participants described the fungal hyphae as angiocentric. Although there is significant tissue damage surrounding the mass of fungal hyphae it appears to be surrounded by a thin layer of smooth muscle which could represent either the wall of an artery or a bronchiole. Based on the pathogenesis of Aspergillus sp., our morphologic diagnosis is arteritis. Although Aspergillus fumigatus and A. flavus are often associated with chronic destructive bronchitis in German shepherd dogs, in other species they commonly disseminate and spread systemically to other organs. Fungal conidia and hyphae are able to block immune responses and evade killing by phagocytes. Macrophages phagocytize fungi by identification of pathogen associated molecular patterns (PAMPs) using pattern recognition receptors (PRRs) expressed on the surface of macrophages and other phagocytic cells. Specifically, Aspergillus fumigatus uses ?-glucans, melanin, and other molecules to block lysosomal killing by reactive oxygen species, phagolysosomal acidification, and other destructive mechanisms in macrophages and neutrophils to allow for systemic spread via leukocyte trafficking. In addition, hyphae can travel separately by invading endothelial cells lining capillaries and access the circulatory system, break off in the blood stream, attach to endothelium, and invade tissues at distant sites. It is postulated that ligand-receptor interactions determine where the fungal hyphae decide to stop and spread.10
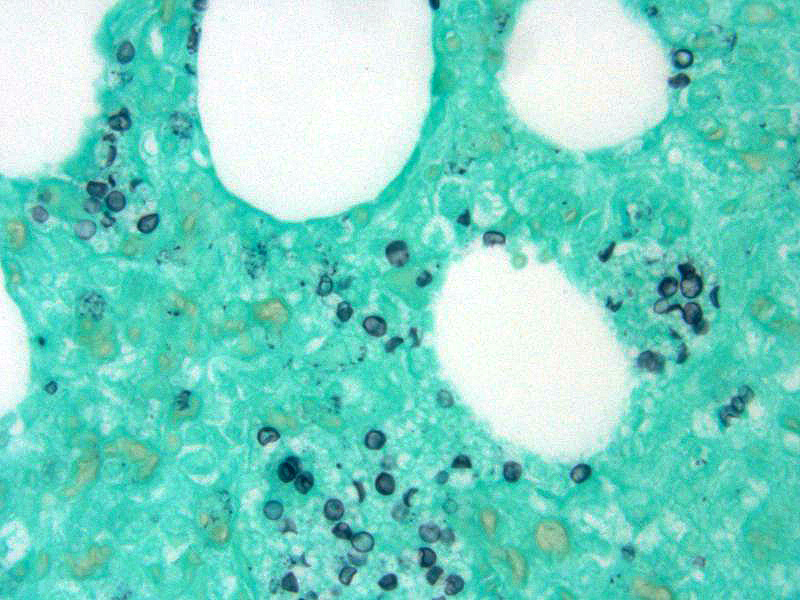
In addition, conference attendees described small, punctate, fungal cysts that partially fill alveoli giving the spaces a honeycombed appearance. These were interpreted as secondary pulmonary pneumocystosis. Pneumocystis carinii (likely P. carinii f. sp. suis) is a common opportunist whose growth is facilitated by immune suppression and has been associated with Porcine Respiratory and Reproductive Syndrome (PRRS) and PCV-2 in pigs. Pneumocystis is a fungus with a small uninucleate trophic form that replicates by binary fission and a larger multinucleate cyst form with 8 intracystic bodies formed by sexual replication. These intracystic bodies are released and attach to type I pneumocytes where they mature into trophic forms. Binding to type I pneumocytes, macrophages, surfactant proteins, and fibronectin is mediated by glycoprotein A (cell surface protein). Grossly, Pneumocystis results in a diffusely firm, rubbery, lung. The characteristic microscopic finding is a soap-bubble material filling alveoli which represents intracellular and extracellular fungal bodies. The wall of the cyst form stains with Gomori methenamine silver (GMS) and Periodic acid-Schiff (PAS).1 In this case, GMS identified numerous Pneumocystis sp. organisms scattered throughout the lung parenchyma often lining alveoli.
Institute of Animal Pathology
Vetsuisse Faculty
University of Bern
Länggassstrasse 122, 3012
Bern, Switzerland
http://www.itpa.vetsuisse.unibe.ch/index_eng.html
References:
1. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer"s Pathology of Domestic Animals. Vol. 2. 6th ed. St. Louis, MI: Elsevier; 2016:527-529, 535-536.
2. Fachinger V, Bischoff R, Jedidia SB, et al. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 2008; 26: 1488-1499.
3. Gerber PF, Johnson J, Shen H, et al. Association of concurrent porcine circovirus (PCV) 2a and 2b infection with PCV associated disease in vaccinated pigs. Res Vet Sci 2013; 95: 775-781.
4. Kixmöller M, Ritzmann M, Eddicks M, et al. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 2008; 26: 3443-3451.
5. López A, Martinson A. Respiratory System, Mediastinum, and Pleurae. In: Zachary JM, ed. Pathologic Basis of Veterinary Disease. Vol. 6th ed. St. Louis, MI: Elsevier; 2017:541.
6. Opriessnig T and Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol 2013; 50: 23-38.
7. Opriessnig T, Meng XJ and Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 2007; 19: 591-615.
8. Pejsak Z, Podgorska K, Truszczynski M, et al. Efficacy of different protocols of vaccination against porcine circovirus type 2 (PCV2) in a farm affected by postweaning multisystemic wasting syndrome (PMWS). Comp Immunol Microbiol Infect Dis 2010; 33: e1-5.
9. Segales J, Urniza A, Alegre A, et al. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine 2009; 27: 7313-7321.
10. Zachary JF, Mechanisms of Microbial Infections. In: Zachary JM, ed. Pathologic Basis of Veterinary Disease. Vol. 6th ed. St. Louis, MI: Elsevier; 2017:219-220, 233-234.