CONFERENCE 23, CASE 2
Signalment:
A 7-year-old spayed male domestic shorthair cat (Felis silvestris catus).
History:
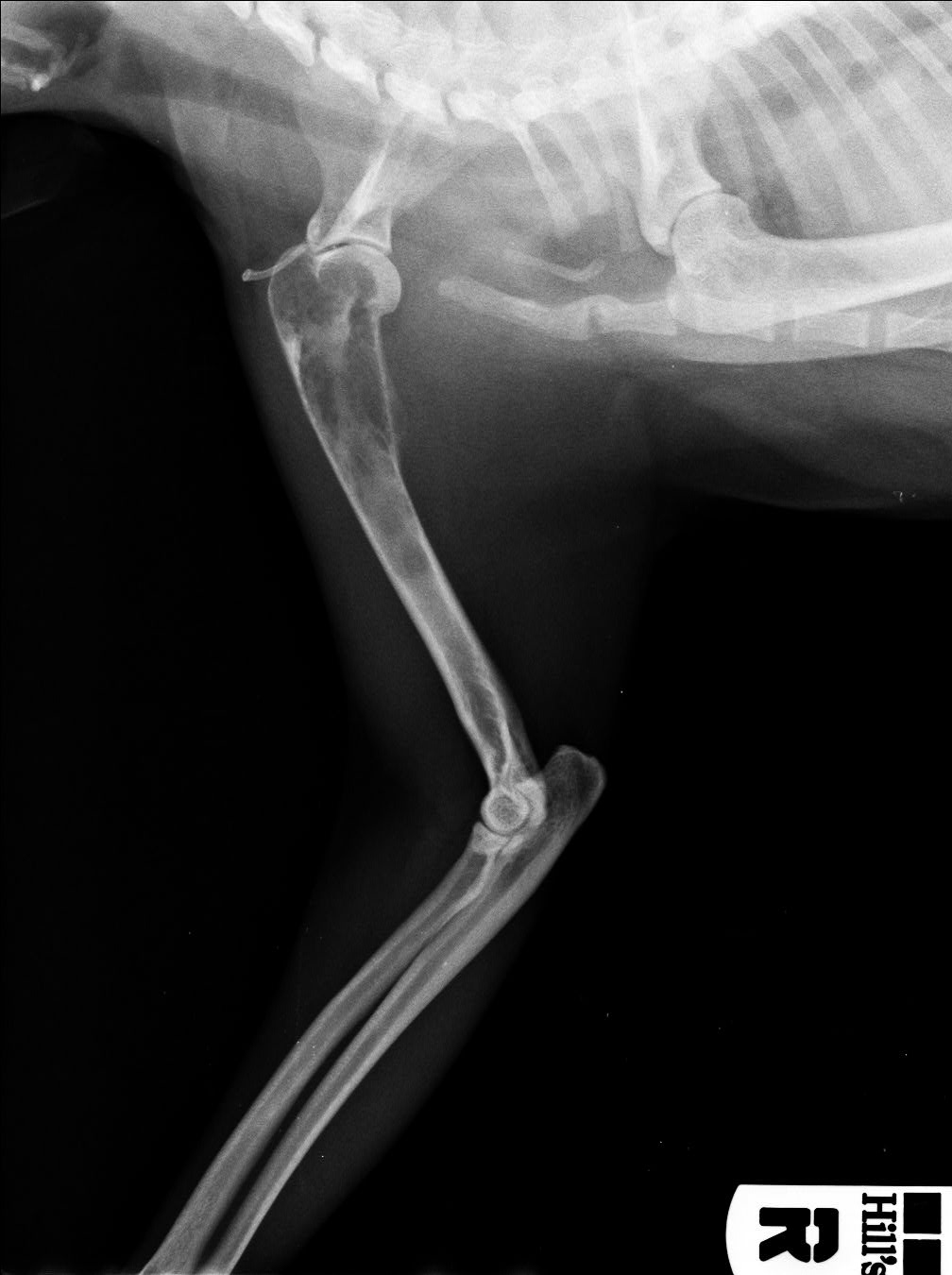
A 7-year-old, spayed male domestic shorthair cat, weighing 4.65 kg (body condition score: 5/9), regularly vaccinated and living indoor, was presented to the Veterinary Clinic for evaluation of grade III lameness in the right forelimb. The owner reported that the lameness was observed for a few days following a trauma. Upon presentation, the cat was bright and alert, but exhibited an aggressive behaviour. Physical examination revealed no abnormalities, muscular asymmetry or atrophy were not observed in the forelimbs. The orthopaedic examination indicated moderate pain reaction upon palpation of the proximal humeral epiphysis and scapulo-humeral joint. The neurological examination was unremarkable. Under anaesthesia, radiography revealed a focally expansile lesion and bone lysis in the right third proximal humerus with evident thinning of the cortical bone extending towards the diaphysis. There was mild focal periosteal reactivity. The lesion was compartmentalized by trabeculae traversing an area of radiolucency, resulting in a mild “soap bubble” appearance. Soft tissue swelling was also observed overlying the region adjacent to the humeral lesion.
Chest and contralateral limbs X-rays were within normal limits. Ultrasound study of adjacent axillary and superficial cervical lymph nodes was also within normal limits.
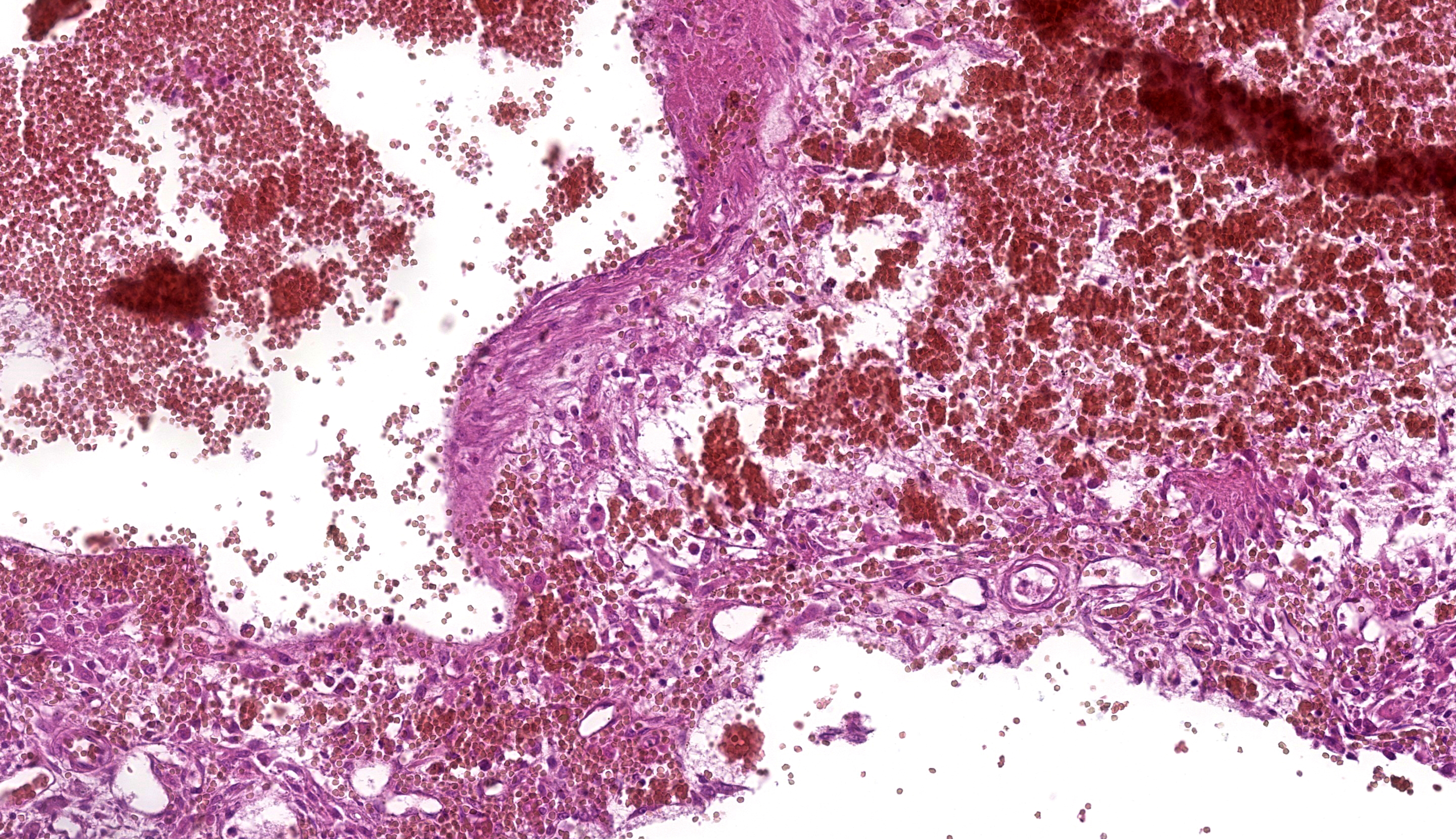
Fine-needle aspiration (FNA) was performed on the bone lesion. The needle easily penetrated the cortical bone into the medullary canal. Cytologic evaluation of FNA revealed hemo-diluted cytological samples with mild cellularity, primarily consisting of haematopoietic elements, small lymphocytes and occasional small aggregates of round to spindle cells with indistinct cells borders and abundant, basophilic cytoplasm, with oval nuclei, reticular chromatin and prominent nucleoli (figures 3 and 4). The anisocytosis and anisokaryosis were moderate. Finally, rare foamy macrophages were observed.
The cytological diagnosis indicated bone marrow contamination due to the sampling procedure. The presence of occasional mesenchymal elements with moderate atypia could suggest a sarcomatous origin of the lesion, for which histopathological confirmation was recommended.
The owner declined the biopsy procedure and provided informed consent for the amputation with scapulectomy and axillary lymph node dissection.
Gross Pathology:
Grossly, an expansile, raised, round nodular lesion measuring about 18 x 12 x 10 mm was present on the third lateral superior proximal humerus. On the cut surface, a cavity full of blood was found within the centre of the bone, expanding the cortex, and internally septated, creating multiple hematic lacunae of variable sizes and cyst-like appearance. A mild periostal thickening, above the cystic lesion was observed. The joints were unremarkable, and no masses were present in the surrounding soft tissue.
Microscopic Description:
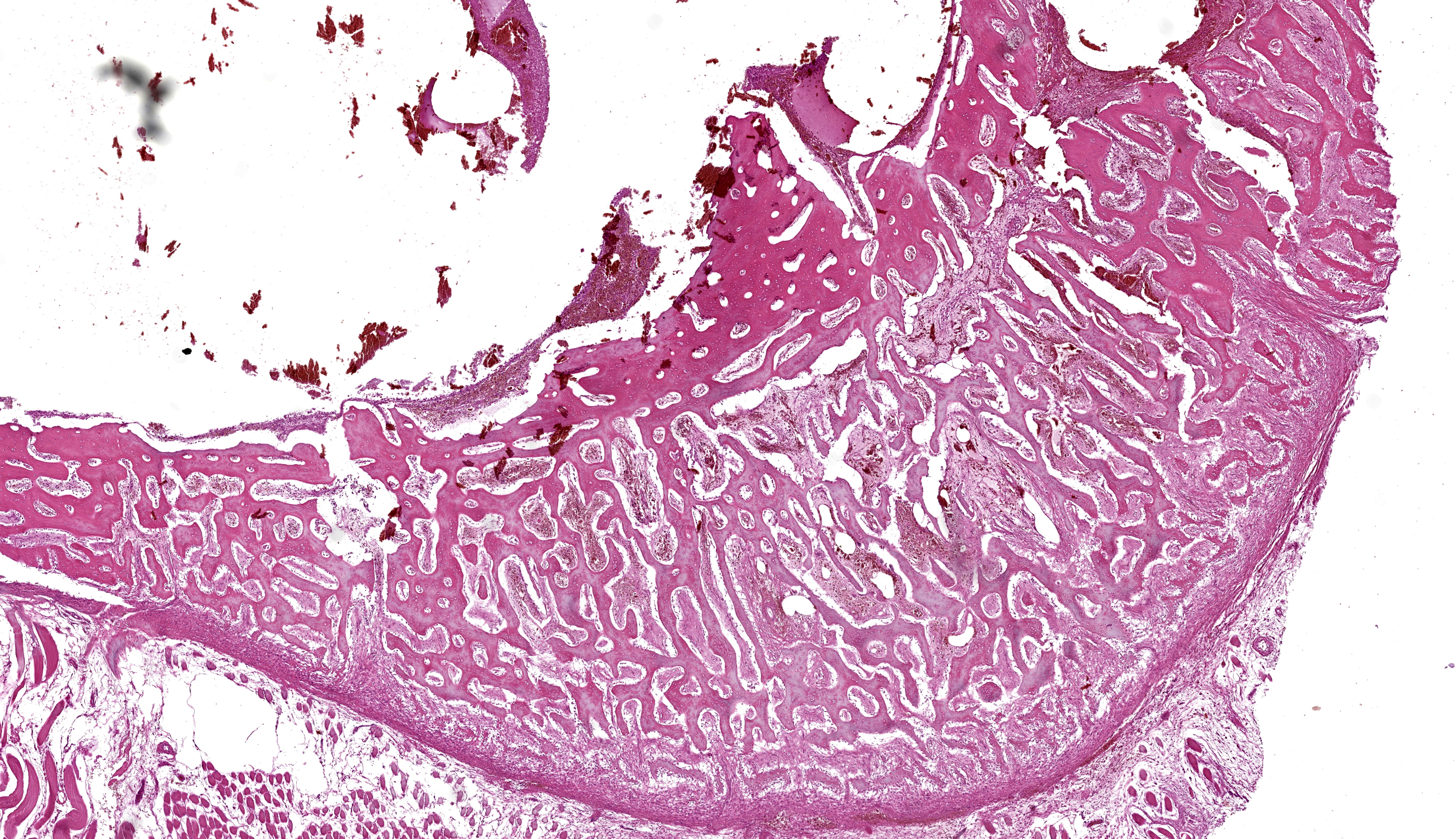
Bone and soft tissue from the periphery of the right proximal humerus. Affecting 70% of the sections and located eccentrically in the bone cavity, involving the medullary cavity, and extending into the cortex, a round, well-demarcated, non-infiltrative, non-capsulated mass composed of multiple blood-filled spaces of variable sizes is observed, measuring about 15 x 10 x 8 mm. These spaces contain numerous extravasated red blood cells admixed with eosinophilic, fibrillar, material (fibrin), eosinophilic amorphous material (serous) and are well delimited by septa of variable thickness leaning on spicules of bone (fig. 5). The septa are composed of moderately cellular proliferation characterized by spindle cells with minimal atypia (possible fibrovascular proliferation), multifocally arranged in solid areas (fig. 6). Admixed, mild to moderate proliferation of round to oval cells with moderate, eosinophilic, cytoplasm and an eccentric, round, nucleus (possibly osteoblasts, fig. 7) was noticed. Scattered osteoclasts and foamy macrophages with abundant, pink to yellow-brown cytoplasm (hemosiderophages) are observed. The trabecular bone affected by the cystic mass shows, multifocally, irregular margins with shallow pits (Howship’s lacunae) occasionally associated with osteoclasts (bone resorption). The rest of bone shows tinctorial alterations, from intense to pale eosinophilic matrix, in which randomly scalloped lines could be observed (new bone deposition). The periosteum appears highly cellular (reactive) while, multifocally, a mild inflammatory infiltrate composed of lymphocytes, plasma cells and rare hemosiderophages is evident. The surrounding skeletal myofibers are separated by optically empty material (edema) and occasionally show a decreased diameter (mild atrophy).
Contributor’s Morphologic Diagnosis:
Humerus: Focal, monolateral, aneurysmal bone cyst with bone remodelling
Contributor’s Comment: Aneurysmal bone cysts (ABCs) are benign, locally expanding bone masses, containing numerous blood-filled or serosanguineous fluid-filled spaces, not usually lined by endothelium, located between bone trabeculae.8,11 Adjacent tissue to the spaces can vary from well-differentiated fibrous or fibro-osseous tissue to pronounced proliferation of undifferentiated mesenchymal cells admixed with osteoclast-like multinucleated giant cells. ABCs are often locally aggressive, tending to cause lytic bone lesions12 and destruction of the inner cortical bone layers during expansion.8 Haemorrhages and hemosiderosis are frequent.8
The causes and pathogenesis of ABCs remain unknown;8 however, they could be consequences of ischemic necrosis, haemorrhage, disruption or shunting of intramedullary blood vessels, or congenital/acquired vascular malformations.8,11 Local alteration in blood flow has also been thought to play a role.3 Increased venous pressure may occur secondary to trauma or a tumor, resulting in dilation of the vascular bed and subsequent erosion of bone.3
ABCs have been reported rarely in dogs, cats, horses, and cattle,3 predominantly occurring in the flat bones of the axial11 and appendicular skeleton.3 Limited cases are reported in animals to establish age or site prevalence.3 In cats, ABCs have been described in the scapula,1,5 pelvis, 9,12 metatarsal bone,7 humerus,4 and rib.2 Radiographically, ABCs appear as expansile, osteolytic lesions contained by a thin, “ballooned” periosteum with an internal “soap-bubble” appearance caused by internal septa. Grossly, ABCs resemble benign bone cysts, telangiectatic osteosarcoma, and hemangiosarcoma.3 ABCs typically exude blood from the cut surface, and may contain solid areas in addition to multiple blood-filled cysts (multiloculated3). Pathologic fracture may be present.3 Complete resection is the treatment of choice when possible, and carries a favourable prognosis.12 Other treatment options include surgical curettage with bone grafting, and radiation therapy.11 Recurrence after complete surgical excision of the aneurysmal bone cyst (ABC) has not been reported in animals. However, malignant transformation of an aneurysmal bone cyst to a chondrosarcoma has been reported in a dog.3 In humans, ABC is now considered as a benign locally destructive bone neoplasm.6
ABCs must be differentiated from both benign and malignant lesions,10 such as unicameral bone cyst (UBC),12 osteosarcoma, hemangiosarcoma, fibrosarcoma, and plasma cell myeloma.3 One of the major histologic differential diagnosis is telangiectatic osteosarcoma.1,10 However, we excluded it because nuclear pleomorphism and a high mitotic rate were not features of mesenchymal/associated cells in our case. For the same reason, we have excluded fibrosarcoma, plasma cell myeloma, and hemangiosarcoma. Moreover, we have excluded UBC because it is unicameral, while ABCs are multiloculated3 as in our case. Finally, fungal infection and osteomyelitis were ruled out due to negative Periodic acid–Schiff (PAS) staining and the very mild inflammatory infiltration. Indeed, acute osteomyelitis are characterized by the presence of fibrin and a dense population of neutrophils and necrotic cells in the primary spongiosa while the presence of neutrophils and plasma cells within the reactive bone and connective tissue supports a diagnosis of chronic osteomyelitis,3 all features not detected in our case.
Contributing Institution:
Department of Comparative Biomedicine and Food Science, Viale dell’Università 15, 35020, Legnaro (PD), Italy; https://www.bca.unipd.it/
JPC Morphologic Diagnosis: Long bone: Simple bone cyst (pseudocyst).
JPC Comment: We greatly appreciate that the contributor of this second case included radiographs and accompanying cytology to support case discussion. Although anatomic pathologists may feel some discomfort in interpretating radiographs, the group (and Dr. Murphy) felt it was important to maintain basic competency in recognizing and describing bony changes and using this information to enhance slide evaluation. We agree with the radiologic assessment of bony lysis with at best a mild periosteal reaction in this case, and not an aggressive lesion, which typically combines both bony proliferation and lysis.
Evaluation of this slide provides some challenge due to processing artifact, due to the large blood-filled spaces. The composition and arrangement of spindle cells led some participants to promote the idea that this process resembled a seroma forming within bone (i.e. a response to trauma). It is clear from the current literature that the genesis remains nebulous however.
Lastly, it wouldn’t be Wednesday Slide Conference without a good semantic argument and this case offered a perfect minor quibble to occupy at least 5 minutes. While the term “aneurysmal bone cyst” is well established in the veterinary literature, it is also worth mentioning that this is itself a misnomer as there is no lining epithelium present. As such, aneurysmal bone pseudocyst probably is more appropriate to the lesion, but we hate making the associated literature even harder to read/categorize. Dr. Murphy muddied the waters further by observing that human literature partially reserves aneurysmal bone cyst for cases with a known genetic component13 – it is unclear if similar circumstances might apply to the cat in this case. We liked the safety of “simple bone cyst” as an umbrella term and the parallels to human nomenclature, and ultimately combined the two in our morphologic diagnosis (and eschewed “aneurysm bone cyst” entirely.
References:
- Benamou J, Lussier B, Alexander K, Gains MJ, Savard C. Use of magnetic resonance imaging and histopathologic findings for diagnosis of an aneurysmal bone cyst in the scapula of a cat. J Am Vet Med Assoc. 2012;240(1):69–74.
- Biller DS, Johnson GC, Birchard SJ, Fingland RB. Aneurysmal bone cyst in a rib of a cat. J Am Vet Med Assoc. 1987;190(9):1193–1195. (PDF not available)
- Craig LE, Dittmer KE, Thompson KG. Chapter 2 - Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 1 (Sixth Edition). W.B. Saunders 2016:16-163.e1.
- Kim K, Kim H, Kim H, et al. Humeral Aneurysmal Bone Cyst in a Cat with Sequential Computed Tomographic Findings. 2022;9(11):594.
- Manca S, Mate de Haro L, Civello A, Martí J. Aneurysmal bone cyst in the scapula of a young cat. 2022;10(4):e459.
- Nasri E, Reith JD. Aneurysmal bone cyst: a review. J Pathol Transl Med. 2023;57(2):81–87.
- Nicetto T, Coltro A, Massari F. Tarsometatarsal stabilization after metatarsal bone amputation for treatment of an aneurysmal bone cyst in a cat. 2021.
- Olson EJ, Carlson CS. Bones, Joints, Tendons, and Ligaments1. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease (Sixth Edition). Mosby 2017:954-1008.e2.
- Saunders JH, Heimann M, Taeymans O, Snaps FR. Aneurysmal bone cyst in the pelvis of a cat. 2003;72(6).
- Stewart HL. Aneurysmal bone cysts as a diagnostic consideration in juvenile patients: Considerations from humans and animals. 2023;35(4):189–193.
- Voss K. 4 - Diseases of bone. In: Montavon PM, Voss K, Langley-Hobbs SJ, eds. Feline Orthopedic Surgery and Musculoskeletal Disease. W.B. Saunders 2009:55–62.
- Winbladh K, Fransson BA, Svensson G, Karlstam E, Uhlhorn M. Aneurysmal bone cyst in the pelvis of a cat: successful outcome of partial iliectomy with limb preservation. 2020;6(2):2055116920974984.
- Ye, Pringle LM, Lau AW, et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010;29(25):3619–29.